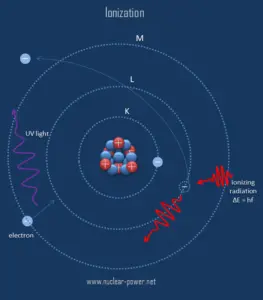
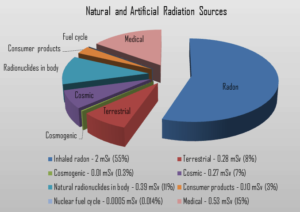
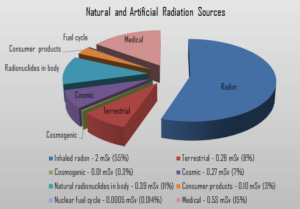
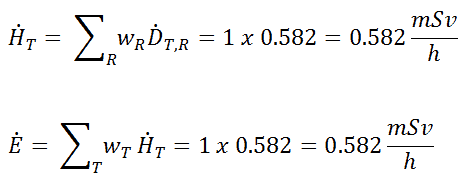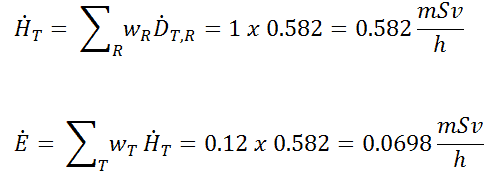 Radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us. It is a part of our natural world that has been here since the birth of our planet. All living creatures, from the beginning of time, have been, and are still being, exposed to ionizing radiation. Ionizing radiation is generated through nuclear reactions, nuclear decay, by very high temperature, or via acceleration of charged particles in electromagnetic fields. But in general, there are two broad categories of radiation sources:
Radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us. It is a part of our natural world that has been here since the birth of our planet. All living creatures, from the beginning of time, have been, and are still being, exposed to ionizing radiation. Ionizing radiation is generated through nuclear reactions, nuclear decay, by very high temperature, or via acceleration of charged particles in electromagnetic fields. But in general, there are two broad categories of radiation sources:
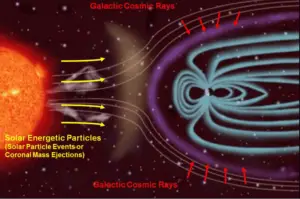
- Natural Background Radiation. Natural background radiation includes radiation produced by the Sun, lightnings, primordial radioisotopes or supernova explosions etc.
- Man-Made Sources of Radiation. Man-made sources include medical uses of radiation, residues from nuclear tests, industrial uses of radiation etc.
Special Reference: Sources and effects of ionizing radiation, Annex B. UNSCEAR. New York, 2010. ISBN: 978-92-1-142274-0.
Man-made Sources of Radiation
Since ionizing radiation has many industrial, and medical uses, people can be exposed also to man-made sources of radiation. Man-made sources include medical uses of radiation, residues from nuclear tests, industrial uses of radiation, television, and numerous other radiation producing devices. For example, in some kind of smoke detectors, you can meet man-made radionuclides such as americium-241. This man-made radionuclide is used to ionize air and to detect smoke.
It must be noted, most of these exposures are very low in intensity and the total dose and does not posse larger health effects. In each case, usefulness of ionizing radiation must be balanced with its hazards. Nowadays a compromise was found and most of uses of radiation are optimized. Today it is almost unbelievable that x-rays was, at one time, used to find the right pair of shoes (i.e. shoe-fitting fluoroscopy). Measurements made in recent years indicate that the doses to the feet were in the range 0.07 – 0.14 Gy for a 20 second exposure. This practice was halted when the risks of ionizing radiation were better understood.
There are two distinct groups exposed to man-made radiation sources. The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) itemized types of human exposures as:
- public exposure, which is the exposure of individual members of the public and of the population in general
- occupational radiation exposure, which is the exposure of workers in situations where their exposure is directly related to or required by their work
Public Exposure
In general, the following man-made sources expose the public to radiation:
- Medical Exposures (by far, the most significant man-made source)
- Diagnostic x-rays
- Nuclear medicine procedures (iodine-131, cesium-137, technetium-99m etc.)
- Consumer Products
- Building and road construction materials
- Smoking cigarettes (polonium-210)
- Combustible fuels, including gas and coal
- X-ray security systems
- Televisions
- Smoke detectors (americium)
- Lantern mantles (thorium)
To a lesser degree, the public is also exposed to radiation from the nuclear fuel cycle, from uranium mining and milling to disposal of used (spent) fuel. Noteworthy, the public is also exposed to radiation from so called “enhanced sources of naturally occurring radioactive material”. This means also industries such as metal mining, coal mining and power production from coal creates additional exposures due to densification of naturally occurring radionuclides. The public receives some minimal exposure from the transportation of radioactive materials and fallout from nuclear weapons testing and reactor accidents (such as Chernobyl).
For that reason, most regulatory bodies require to limit the maximum radiation exposure to individual members of the public to 100 mrem (1 mSv) per year.
Occupational Exposure
As was written, occupational exposure is the exposure of workers in situations where their exposure is directly related to or required by their work. According to ICRP, occupational exposure refers to all exposure incurred by workers in the course of their work, with the exception of
- excluded exposures and exposures from exempt activities involving radiation or exempt sources
- any medical exposure
- the normal local natural background radiation.
In general, occupationally exposed individuals work in the following areas:
- Fuel cycle facilities
- Industrial radiography
- Radiology departments (medical)
- Nuclear medicine departments
- Radiation oncology departments
- Nuclear power plants
- Government and university research laboratories
Such individuals are exposed to varying types and amounts of radiation, depending on their specific jobs and the sources with which they work. For that reason, most regulatory bodies require to limit occupational exposure to adults working with radioactive material to 5000 mrem (50 mSv) per year. Toward that end, employers carefully monitor the exposure of these individuals using instruments called dosimeters worn on a position of the body representative of its exposure. In most situations of occupational exposure the effective dose, E, can be derived from operational quantities using the following formula:

The committed dose is a dose quantity that measures the stochastic health risk due to an intake of radioactive material into the human body.
See also: Dose Monitoring
Medical Exposures – Doses from Medical Radiation Sources
Radiation is used in variety of medical examinations and treatments. Doses from medical radiation sources are naturally determined, whether a person underwent a treatment or not. In general, radiation exposures from medical diagnostic examinations are low (especially in diagnostic uses). Doses may be also high (only for therapeutic uses), but in each case, they must be always justified by the benefits of accurate diagnosis of possible disease conditions or by benefits of accurate treatment. These doses include contributions from medical and dental diagnostic radiology (diagnostic X-rays), clinical nuclear medicine and radiation therapy.
The medical use of ionizing radiation remains a rapidly changing field. In any case, usefulness of ionizing radiation must be balanced with its hazards. Nowadays a compromise was found and most of uses of radiation are optimized. Today it is almost unbelievable that x-rays was, at one time, used to find the right pair of shoes (i.e. shoe-fitting fluoroscopy). Measurements made in recent years indicate that the doses to the feet were in the range 0.07 – 0.14 Gy for a 20 second exposure. This practice was halted when the risks of ionizing radiation were better understood.
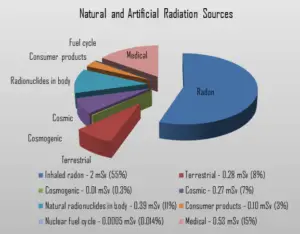
In the following points we try to express enormous ranges of radiation exposure as well as a few doses from medical sources.
- 1 µSv – Eating one banana
- 1 µSv – Extremity (hand, foot, etc.) X-ray
- 5 µSv – Dental X-ray
- 10 µSv – Average daily dose received from natural background
- 40 µSv – A 5-hour airplane flight
- 100 µSv – Chest X-ray
- 600 µSv – mammogram
- 1 000 µSv – Dose limit for individual members of the public, total effective dose per annum
- 3 650 µSv – Average yearly dose received from natural background
- 5 800 µSv – Chest CT scan
- 10 000 µSv – Average yearly dose received from natural background in Ramsar, Iran
- 20 000 µSv – single full-body CT scan
- 80 000 µSv – The annual local dose to localized spots at the bifurcations of segmental bronchi in the lungs caused by smoking cigarettes (1.5 packs/day).
- 175 000 µSv – Annual dose from natural radiation on a monazite beach near Guarapari, Brazil.
- 5 000 000 µSv – Dose that kills a human with a 50% risk within 30 days (LD50/30), if the dose is received over a very short duration.
As can be seen, low-level doses are common for everyday life.
Tobacco – Smoking Cigarettes – Radiation Dose
In addition to chemical, nonradioactive carcinogens, tobacco and tobacco smoke contain small amounts of lead-210 and polonium-210 both of which are radioactive carcinogens. It must be emphasized, cigarettes and tobacco contain also polonium-210, originating from the decay products of radon, which stick to tobacco leaves. Polonium-210 emits a 5.3 MeV alpha particle, which provides most of equivalent dose. Due to decay of polonium-210, the annual local dose to localized spots at the bifurcations of segmental bronchi in the lungs caused by smoking cigarettes (1.5 packs/day) is about 80 mSv/year. Heavy smoking results in a dose of 160 mSv/year. This dose is not readily comparable to the radiation protection limits, since the latter deal with whole body doses, while the dose from smoking is delivered to a very small portion of the body. Many researchers believe that doses from polonium-210 are the origin of the high incidence of lung cancer among smokers.
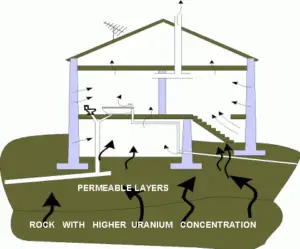
Recall, lead-210 and polonium-210 are daughter nuclei of radon-222. Radon-222 is a gas produced by the decay of radium-226. Both are a part of the natural uranium series. Since uranium is found in soil throughout the world in varying concentrations, also dose from gaseous radon is varying throughout the world. Radon-222 is the most important and most stable isotope of radon. It has a half-life of only 3.8 days, making radon one of the rarest elements since it decays away so quickly. An important source of natural radiation is radon gas, which seeps continuously from bedrock but can, because of its high density, accumulates at the ground. The fact radon is gas plays a crucial role in spreading of all its daughter nuclei. As radon-222 decays into lead-210, lead-210 can be attached to dust of moisture particles and it can be sticked to tobacco leaves. When these particles are concentrated by smoking and inhaled as smoke, some of lead-210 is retained by the body. Since lead-210 is a weak beta emitter, it does not cause major doses, but polonium-210 does.
See also: Radon – Health Effects
The polonium-210, the decay product of lead-210, emits a 5.3 MeV alpha particle, which provides most of equivalent dose. Alpha particles, that belongs to high-LET radiation, are fairly massive and carry a double positive charge, so they tend to travel only a short distance and do not penetrate very far into tissue if at all. However alpha particles will deposit their energy over a smaller volume (possibly only a few cells if they enter a body) and cause more damage to those few cells (more than 80 % of the absorbed energy from radon is due to the alpha particles). Therefore, the radiation weighting factor for alpha radiation is equal to 20. An absorbed dose of 1 mGy by alpha particles will lead to an equivalent dose of 20 mSv.
Special Reference: Sources and effects of ionizing radiation, Annex B. UNSCEAR. New York, 2010. ISBN: 978-92-1-142274-0.
Nuclear Fallout – Radiation Doses
In general, nuclear fallout is the residual radioactive material from a nuclear blast that “falls out” of the sky after an atmospheric explosion. Fallout can also refer to nuclear reactor accidents, although a nuclear reactor does not explode like a nuclear weapon. The isotopic signature of fallout from nuclear blast is very different from the fallout from a serious power reactor accident.
In case of radiation doses from fallout, we consider the residual radioactive material from nuclear tests (not from reactor accidents) that were performed particularly in the two periods from 1954 to 1958 and from 1961 to 1962. According to the UNSCEAR, about 502 atmospheric tests, with a total fission and fusion yield of 440 Mt, were conducted.
Special Reference: Sources and effects of ionizing radiation, Annex B. UNSCEAR. New York, 2010. ISBN: 978-92-1-142274-0.
Fallout from a nuclear tests consist of fission fragments and neutron activation products. When a blast takes place on the ground or in the atmosphere near the ground, large amounts of activation products are formed also from surface materials. The fallout is particularly significant in the neighborhood of the test site, since the larger particles and most of debris lands on the ground (local fallout). But smaller particles may remain aloft in the upper atmosphere for years. These particles can be therefore distributed nearly uniformly around the world, and contribute to so called global fallout. Equivalent doses from global fallout dropped from about 130 μSv/year in 1963 to about 10 μSv/year in recent years.
Radiation Exposures from Electricity Generation
In this chapter, we would like to discuss a very interesting fact. It is generally known, the increasing use of nuclear power and electricity generation using nuclear reactors will lead to a small but increasing radiation dose to the general public. But it is not generally known, power generation from coal also creates additional exposures, and, what is more interesting, while exposure levels are very low, the coal cycle contributes more than half of the total radiation dose to the global population from electricity generation. The nuclear fuel cycle contributes less than one-fifth of this. The collective dose, which are defined as the sum of all individual effective doses in a group of people over the time period or during the operation being considered due to ionizing radiation, is:
- 670-1400 man Sv for coal cycle, depending on the age of the power plant,
- 130 man Sv for nuclear fuel cycle,
- 5-160 man Sv for geothermal power,
- 55 man Sv for natural gas
- 0.03 man Sv for oil
Yes, these results should be seen from the perspective of the share of each technology in worldwide electricity production. Since 40 per cent of the world’s energy was produced by the coal cycle in 2010, and 13 per cent by nuclear, the normalized collective dose will be about the same:
- 0.7 – 1.4 man Sv/GW.a (man sievert per gigawatt year) for coal cycle
- 0.43 man Sv/GW.a (man sievert per gigawatt year) for nuclear fuel cycle
Special Reference: Sources and effects of ionizing radiation, UNSCEAR 2016 – Annex B. New York, 2017. ISBN: 978-92-1-142316-7.
The above doses are related to public exposure. If we consider occupational exposure, regarding the mining of rare earth metals needed for construction, by far the largest collective dose to workers per unit of electricity generated assessed by the UNSCEAR came from solar power, followed by wind power. For solar power, occupational collective dose normalized to energy is a factor of forty and eighty larger than for the nuclear fuel cycle and coal cycle, respectively.
Note that, the collective effective dose is often used to estimate the total health effects, but according to the ICRP this should be avoided (see more: Collective Dose).
![]() In the semiconductor, free charge carriers are electrons and electron holes (electron-hole pairs). Electrons and holes are created by excitation of electron from valence band to the conduction band. An electron hole (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or atomic lattice. It is one of the two types of charge carriers that are responsible for creating electric current in semiconducting materials. Since in a normal atom or crystal lattice the negative charge of the electrons is balanced by the positive charge of the atomic nuclei, the absence of an electron leaves a net positive charge at the hole’s location. Positively charged holes can move from atom to atom in semiconducting materials as electrons leave their positions. When an electron meets with a hole, they recombine and these free carriers effectively vanish. The recombination means an electron which has been excited from the valence band to the conduction band falls back to the empty state in the valence band, known as the holes.
In the semiconductor, free charge carriers are electrons and electron holes (electron-hole pairs). Electrons and holes are created by excitation of electron from valence band to the conduction band. An electron hole (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or atomic lattice. It is one of the two types of charge carriers that are responsible for creating electric current in semiconducting materials. Since in a normal atom or crystal lattice the negative charge of the electrons is balanced by the positive charge of the atomic nuclei, the absence of an electron leaves a net positive charge at the hole’s location. Positively charged holes can move from atom to atom in semiconducting materials as electrons leave their positions. When an electron meets with a hole, they recombine and these free carriers effectively vanish. The recombination means an electron which has been excited from the valence band to the conduction band falls back to the empty state in the valence band, known as the holes.