Gadolinium was first discovered in 1880 by Jean Charles Galissard de Marignac. It is named for mineral (gadolinite), which is named for Finnish chemist Johan Gadolin.
Natural gadolinium consists of six stable isotopes, 154Gd (2.18%), 155Gd (14.8%), 156Gd (20.5%), 157Gd (15.7%), 158Gd (24.8%) and 160Gd (21.9%) and one radioisotope 152Gd (0.2%) with half-life of 1.1 x 1014 y.
In nuclear industry gadolinium is commonly used as a neutron absorber due to very high neutron absorbtion cross-section of two isotopes 155Gd and 157Gd. In fact their absorption cross-sections are the highest among all stable isotopes. 155Gd has 61 000 barns for thermal neutrons (for 0.025 eV neutron) and 157Gd has even 254 000 barns. For this reason gadolinium is widely used as a burnable absorber, which is commonly used in fresh fuel to compensate an excess of reactivity of reactor core. In comparison with another burnable absorbers gadolinium behaves like a completely black material. Therefore gadolinium is very effective in compensation of the excess of reactivity, but on the other hand an improper distribution of Gd-burnable absorbers may lead to unevenness of neutron-flux density in the reactor core.
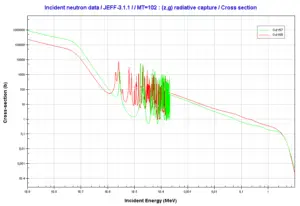
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
We hope, this article, Gadolinium, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.