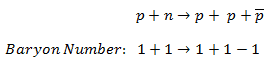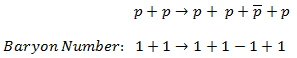What is Proton – Properties of Proton
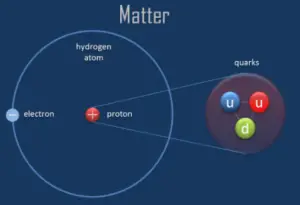 A proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter. It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10−27 kg (938.272 MeV/c2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. The proton has a mean square radius of about 0.87 × 10−15 m, or 0.87 fm, and it is a spin – ½ fermion.
A proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter. It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10−27 kg (938.272 MeV/c2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. The proton has a mean square radius of about 0.87 × 10−15 m, or 0.87 fm, and it is a spin – ½ fermion.
The protons exist in the nuclei of typical atoms, along with their neutral counterparts, the neutrons. Neutrons and protons, commonly called nucleons, are bound together in the atomic nucleus, where they account for 99.9 percent of the atom’s mass. Research in high-energy particle physics in the 20th century revealed that neither the neutron nor the proton is not the smallest building block of matter.
Structure of Proton
Protons and neutrons have also their structure. Inside the protons and neutrons, we find true elementary particles called quarks. Within the nucleus, protons and neutrons are bound together through the strong force, a fundamental interaction that governs the behaviour of the quarks that make up the individual protons and neutrons.
The proton has a quark composition of uud, and so its charge quantum number is:
q(uud) = ⅔ + ⅔ + (-1/3) = +1
The mass of the proton is 938.272 MeV/c2, whereas the mass of the three quarks is only about 12 MeV/c2 (only about 1% of the mass-energy of the neutron). Like the proton, most of mass (energy) of the neutron is in the form of the strong nuclear force energy (gluons). The quarks of the neutron are held together by gluons, the exchange particles for the strong nuclear force.
Noteworthy, because most of your mass is due to the protons and neutrons in your body, your mass (and therefore your weight on a bathroom scale) comes primarily from the gluons that bind the constituent quarks together, rather than from the quarks themselves. Mass is primarily a measure of the energies of the quark motion and the quark-binding fields any real object. It must be noted, gluons are inherently massless, they possess energy.
Stability of Proton
The free proton (a proton not bound to nucleons or electrons) is a stable particle that has not been observed to break down spontaneously to other particles. Free protons are found naturally in a number of situations (e.g. they make up 90% of cosmic rays) in which energies or temperatures are high enough to separate them from electrons, for which they have some affinity.
Decay of proton is also associated with the law of conservation of baryon number. Baryon number is a generalization of nucleon number, which is conserved in nonrelativistic nuclear reactions and decays. The law of conservation of baryon number states that:
The sum of the baryon number of all incoming particles is the same as the sum of the baryon numbers of all particles resulting from the reaction.
For example, the following reaction has never been observed:
even if the incoming proton has sufficient energy and charge, energy, and so on, are conserved. This reaction does not conserve baryon number since the left side has B =+2, and the right has B =+1.
On the other hand, the following reaction (proton-antiproton pair production) does conserve B and does occur if the incoming proton has sufficient energy (the threshold energy = 5.6 GeV):
As indicated, B = +2 on both sides of this equation.
From these and other reactions, the conservation of baryon number has been established as a basic principle of physics. This principle provides basis for the stability of the proton. Since the proton is the lightest particle among all baryons, the hypothetical products of its decay would have to be non-baryons. Thus, the decay would violate the conservation of baryon number. It must be added some theories have suggested that protons are in fact unstable with very long half-life (~1030years) and that they decay into leptons. There is currently no experimental evidence that proton decay occurs.
Antiproton
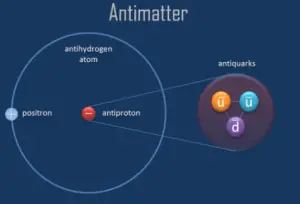 As was written, a particle and its antiparticle have the same mass as one another, but opposite electric charge, and other differences in quantum numbers. That means a proton has positive charge while an antiproton has negative charge and therefore they attract each other. A collision between any particle and its antiparticle partner is known to lead to their mutual annihilation. Since matter and antimatter carry an immense amount of energy (due to E = mc2), their mutual annihilation is associated with production of intense photons (gamma rays), neutrinos, and sometimes less-massive particle–antiparticle pairs.
As was written, a particle and its antiparticle have the same mass as one another, but opposite electric charge, and other differences in quantum numbers. That means a proton has positive charge while an antiproton has negative charge and therefore they attract each other. A collision between any particle and its antiparticle partner is known to lead to their mutual annihilation. Since matter and antimatter carry an immense amount of energy (due to E = mc2), their mutual annihilation is associated with production of intense photons (gamma rays), neutrinos, and sometimes less-massive particle–antiparticle pairs.
Protons and Neutrons in a Nucleus
 A nuclear stability is determined by the competition between two fundamental interactions. Protons and neutrons are attracted each other via residual strong force. On the other hand protons repel each other via the electric force due to their positive charge. Therefore neutrons within the nucleus act somewhat like nuclear glue, neutrons attract each other and protons , which helps offset the electrical repulsion between protons. There are only certain combinations of neutrons and protons, which forms stable nuclei. For example, the most common nuclide of the common chemical element lead (Pb) has 82 protons and 126 neutrons.
A nuclear stability is determined by the competition between two fundamental interactions. Protons and neutrons are attracted each other via residual strong force. On the other hand protons repel each other via the electric force due to their positive charge. Therefore neutrons within the nucleus act somewhat like nuclear glue, neutrons attract each other and protons , which helps offset the electrical repulsion between protons. There are only certain combinations of neutrons and protons, which forms stable nuclei. For example, the most common nuclide of the common chemical element lead (Pb) has 82 protons and 126 neutrons.
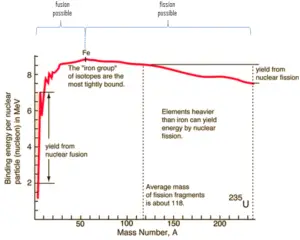
Because of the strength of the nuclear force at short distances, the nuclear binding energy (the energy required to disassemble a nucleus of an atom into its component parts) of nucleons is more than seven orders of magnitude larger than the electromagnetic energy binding electrons in atoms. Nuclear reactions (such as nuclear fission or nuclear fusion) therefore have an energy density that is more than 10 000 000x that of chemical reactions.
We hope, this article, Proton – Properties of Proton, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.