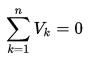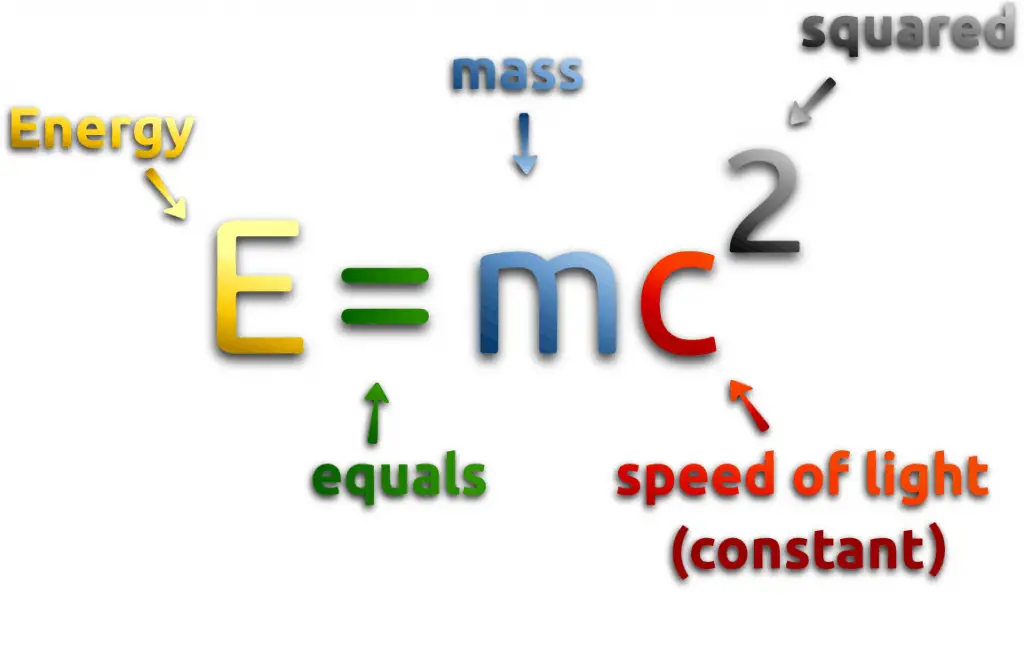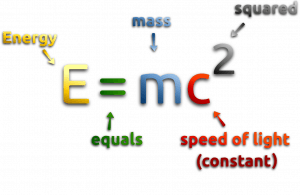What is Energy
Source: hyperphysics.phy-astr.gsu.edu
The term energy is very very broad and it has many definitions. Technically, energy is a scalar physical quantity that is associated with the state of one or more objects. Energy is generally defined as the potential to do work or produce heat. Sometimes it is like the “currency” for performing work. You must have energy to accomplish work. To do 1 kilojoule of work, you must expend 1 kilojoule of energy. It must be added, this interpretation can be misleading because energy is not necessarily available to do work.
One of the most wonderful properties of the universe is that energy can be transformed from one type to another and transferred from one object to another. Moreover, when transformed from one type to another and transferred from one object to another, the total amount of energy is always the same. It is one of the elementary properties of the universe.
For example, burning gasoline to power cars is an energy conversion process we rely on. The chemical energy in gasoline is converted to thermal energy, which is then converted to mechanical energy that makes the car move. The mechanical energy has been converted to kinetic energy. When we use the brakes to stop a car, that kinetic energy is converted by friction back to heat, or thermal energy.
Energy Units
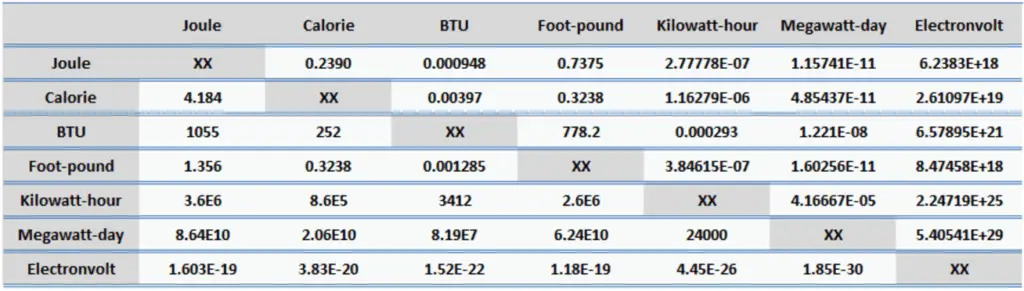
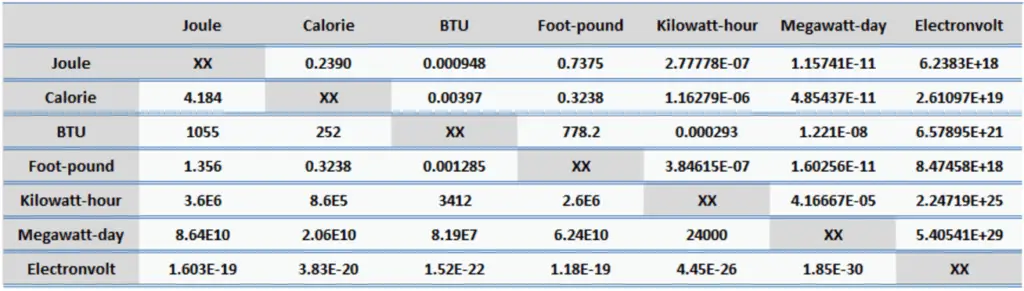
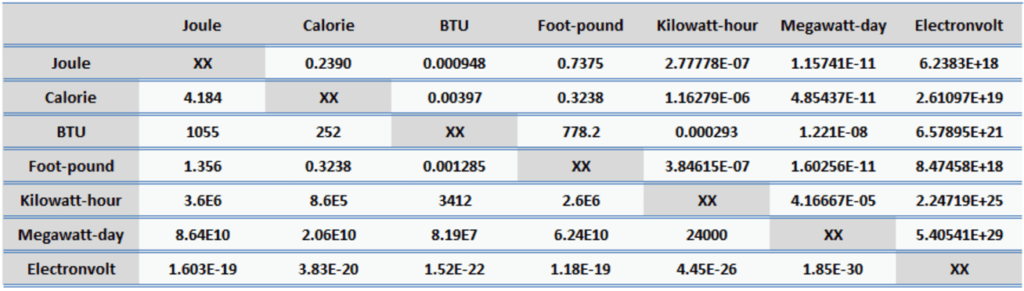
Energy is generally defined as the potential to do work or produce heat. This definition causes the SI unit for energy is the same as the unit of work – the joule (J). Joule is a derived unit of energy and it is named in honor of James Prescott Joule and his experiments on the mechanical equivalent of heat. In more fundamental terms, 1 joule is equal to:
1 J = 1 kg.m2/s2
Since energy is a fundamental physical quantity and it is used in various physical and engineering branches, there are many units in physics and engineering. These units are summarized in following points:
Examples of Energy of 1 Joule
One joule in everyday life and in science corresponds to approximately:
- The kinetic energy of an object with mass 1 kg moving at √2 ≈ 1.4 m/s.
- The kinetic energy of a 50 kg object (e.g. human) moving very slowly – approximately 0.72 km/h.
- The energy required to lift a medium-size apple (100 g) 1 meter vertically from the surface of the Earth.
- The heat required to raise the temperature of 1 g of water by 0.24 °C.
- The heat required to evaporate of 0.00044 g of liquid water at 100°C.
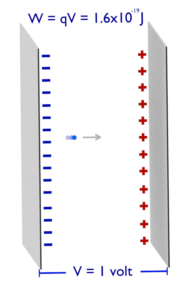
- The amount of electricity required to light a 1 watt LED for 1 s.
- Is released by approximately 3.1⋅1010 fissions in a nuclear reactor.
Forms of Energy
Energy exists in many forms. Common energy forms include mechanical energy that is classically divided into kinetic and potential energy. The kinetic energy is related to the velocity of a moving object. The potential energy is related to an object’s position in a force field (gravitational, electric or magnetic). Tension in a spring or surface film tension are other forms of potential mechanical energy (elastic energy). There are many other forms of energy including electrical, magnetical, chemical, and nuclear energy.
In thermodynamics the concept of energy is broadened to account for other observed changes. Thermodynamics deals with another type of energy called “thermal energy” or “internal energy”. The only ways the energy of a closed system can be changed are through transfer of energy by work or by heat. Further, based on the experiments of Joule and others, a fundamental aspect of the energy concept is that energy is conserved. This principle is known as the first law of thermodynamics. In general energy is a fundamental concept of thermodynamics and one of the most significant aspects of engineering analysis.
Mass-energy Equivalence
One of the striking results of Einstein’s theory of relativity is that mass and energy are equivalent and convertible, one into the other. Equivalence of the mass and energy is described by Einstein’s famous formula:
, where M is the small amount of mass and C is the speed of light.
What that means? If the nuclear energy is generated (splitting atoms, nuclear fusion), a small amount of mass (saved in the nuclear binding energy) transforms into the pure energy (such as kinetic energy, thermal energy, or radiant energy).
The energy equivalent of one gram (1/1000 of a kilogram) of mass is equivalent to:
- 89.9 terajoules
- 25.0 million kilowatt-hours (≈ 25 GW·h)
- 21.5 billion kilocalories (≈ 21 Tcal)
- 85.2 billion BTUs
or to the energy released by combustion of the following:
- 21.5 kilotons of TNT-equivalent energy (≈ 21 kt)
- 568,000 US gallons of automotive gasoline
Any time energy is generated, the process can be evaluated from an E = mc2 perspective.
Principle of Conservation of Energy
One of the most wonderful properties of the universe is that energy can be transformed from one type to another and transferred from one object to another. Moreover, when transformed from one type to another and transferred from one object to another, the total amount of energy is always the same. It is one of the elementary properties of the universe.
In thermodynamics the concept of energy is broadened to account for other observed changes, and the principle of conservation of energy is extended to include a wide variety of ways in which systems interact with their surroundings. The only ways the energy of a closed system can be changed are through transfer of energy by work or by heat. Further, based on the experiments of Joule and others, a fundamental aspect of the energy concept is that energy is conserved. This principle is known as the first law of thermodynamics. The first law of thermodynamics can be written in various forms:
In words:
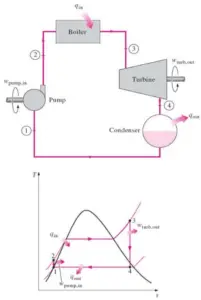
Equation form:
∆Eint = Q – W
where Eint represents the internal energy of the material, which depends only on the material’s state (temperature, pressure, and volume). Q is the net heat added to the system and W is the net work done by the system. We must be careful and consistent in following the sign conventions for Q and W. Because W in the equation is the work done by the system, then if work is done on the system, W will be negative and Eint will increase.
Similarly, Q is positive for heat added to the system, so if heat leaves the system, Q is negative. This tells us the following: The internal energy of a system tends to increase if heat is absorbed by the system or if positive work is done on the system. Conversely, the internal energy tends to decrease if heat is lost by the system or if negative work is done on the system. It must be added Q and W are path dependent, while Eint is path independent.
Differential form:
dEint = dQ – dW
The internal energy Eint of a system tends to increase if energy is added as heat Q and tends to decrease if energy is lost as work W done by the system.
Energy Sources
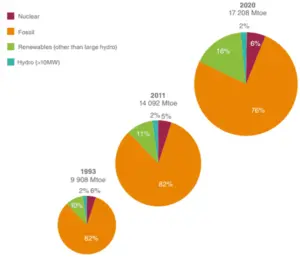
Source: World Energy Resources – 2013 Survey
Used by permission of the World Energy Council
Energy sources have always played a very important role in the development of human society. Since the industrial revolution the energy has been a driving force for the modern civilization development. Technological development and consumption of primary energy, along with the increase of the world population are interdependent. In past 20 years, the world around us has changed significantly. Technology has become one of the main drivers of economic and social development. The rapid advancement of Information Technology (IT) all over the world has transformed not only the way we think, but also the way we act. It must be noted that practically all technologies run on electrical energy and therefore the share of electricity is increasing rapidly, faster than Total Primary Energy Supply (TPES – the sum of production and imports subtracting exports and storage changes.).
At present, fossil fuel is still the world’s predominant energy source and its extraction, production and use are not considered to be efficient regardless of the new technologies available to improve its use and extraction. When studying energy resources, we have to distinguish the primary energy sources and secondary energy sources.
Primary Energy Sources
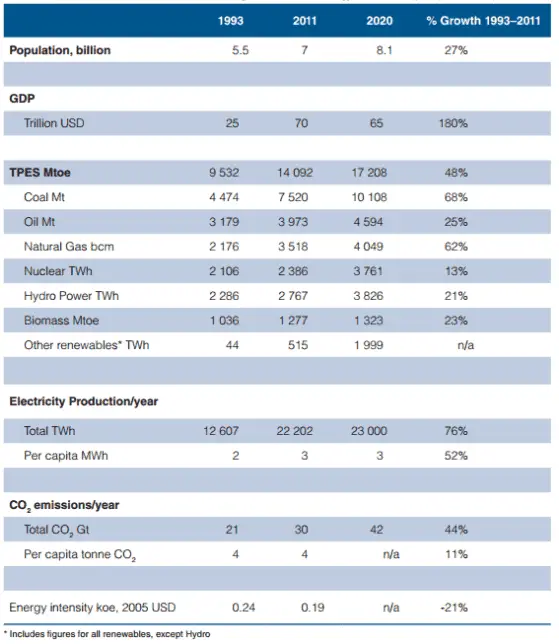
Source: World Energy Resources – 2013 Survey
Used by permission of the World Energy Council
Primary energy (PE) is an energy resource found in nature that has not been subjected to any conversion or transformation process. It is energy contained in raw fuels, and other forms of energy received as input to a system. Primary energy sources take many forms, including nuclear energy, fossil energy — like oil, coal and natural gas — and renewable sources like wind, solar, geothermal and hydropower. These primary sources can be converted to secondary energy source, so called energy carriers. Primary energy sources can be divided into:
- Non-renewable sources
- Fossil fuels
- Oil
- Coal
- Natural gas
- Mineral fuels
- Natural Uranium
- Natural Thorium
- Fossil fuels
- Renewable sources
- Solar energy
- Wind energy
- Hydro and tidal energy
- Geothermal energy
- Biomass energy (if sustainably exploited)
Secondary Energy Sources – Energy Carriers
Secondary energy sources, also called energy carriers, are derived from the transformation of primary energy sources. They are called energy carriers, because they move energy in a useable form from one place to another. The well-known energy carriers are:
- Electricity
- Petrol
- Hydrogen
Electricity and hydrogen made from primary energy sources such as coal, natural gas, nuclear energy, petroleum, and renewable energy sources. Electricity is particularly useful since it has low entropy (is highly ordered) and can be converted into other forms of energy very efficiently. Simply, we cannot say that hydrogen have potential to offset fossil fuels.
Secondary energy sources are used, because its using is easier than using a primary energy source. For example, using electricity for lighting is safer than using petroleum in candles or kerosene lamps.
On the other hand any conversion of primary energy to energy carrier is associated with some inefficiency. Therefore when dealing with secondary energy source, we have to always consider the way, how the carrier was made.
We hope, this article, Energy – Physics, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.