What is Uranium
What is uranium?
Uranium is a naturally-occurring chemical element with atomic number 92 which means there are 92 protons and 92 electrons in the atomic structure. The chemical symbol for uranium is U. Uranium was discovered in 1789 by Martin Klaproth in the mineral called pitchblende (uraninite). He named the newly discovered element after the planet Uranus, which had been discovered eight years earlier. It was first isolated as a metal in 1841 by Eugene-Melchior Peligot. Henri Becquerel discovered uranium to be radioactive in 1896. He discovered that uranium minerals could expose a photographic plate through another material. In fact he was the first to discover the process of radioactivity.
Uranium is commonly found at low levels (a few ppm – parts per million) in all rocks, soil, water, plants, and animals (including humans). Uranium occurs also in seawater, and can be recovered from the ocean water. Significant concentrations of uranium occur in some substances such as uraninite (the most common uranium ore), phosphate rock deposits, and other minerals.
Natural uranium consists primarily of isotope 238U (99.28%), therefore the atomic mass of uranium element is close to the atomic mass of 238U isotope (238.03u). Natural uranium also consists of two other isotopes: 235U (0.71%) and 234U (0.0054%). The abundance of isotopes in the nature is caused by difference in the half-lifes. All three naturally-occurring isotopes of uranium (238U, 235U and 234U) are unstable. On the other hand these isotopes belong to primordial nuclides, because their half-life is comparable to the age of the Earth (~4.5×109 years for 238U). In nuclear reactors we have to consider three artificial isotopes, 236U, 233U and 232U. These are produced by transmutation in nuclear reactors from 235U and 232Th.
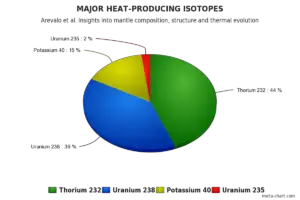 Share of major heat-producing isotopes on the heating of Earth’s core. Uranium 238 has important share of 39%.
Share of major heat-producing isotopes on the heating of Earth’s core. Uranium 238 has important share of 39%.

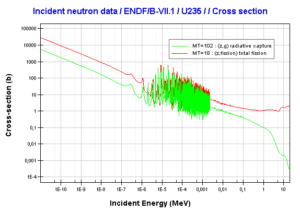 Uranium 235. Comparison of total fission cross-section and cross-section for radiative capture.Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
Uranium 235. Comparison of total fission cross-section and cross-section for radiative capture.Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library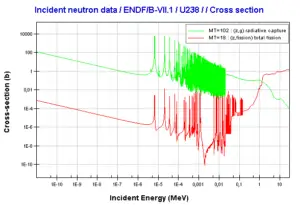 Uranium 238. Comparison of total fission cross-section and cross-section for radiative capture.Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
Uranium 238. Comparison of total fission cross-section and cross-section for radiative capture.Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data LibraryWe hope, this article, Uranium, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.