What is Nuclear Energy
Nuclear energy comes either from spontaneous nuclei conversions or induced nuclei conversions. Among these conversions (nuclear reactions) belong for example nuclear fission, nuclear decay and nuclear fusion. Conversions are associated with mass and energy changes. One of the striking results of Einstein’s theory of relativity is that mass and energy are equivalent and convertible, one into the other. Equivalence of the mass and energy is described by Einstein’s famous formula:
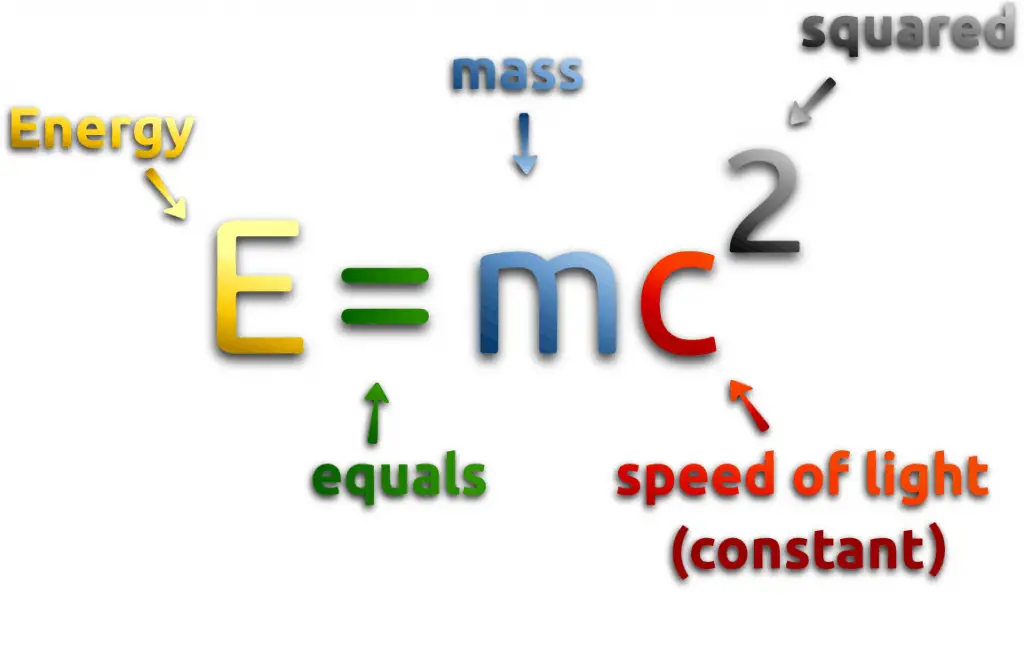
, where M is the small amount of mass and C is the speed of light.
What that means? If the nuclear energy is generated (splitting atoms, nuclear fussion), a small amount of mass (saved in the nuclear binding energy) transforms into the pure energy (such as kinetic energy, thermal energy, or radiant energy).
The energy equivalent of one gram (1/1000 of a kilogram) of mass is equivalent to:
- 89.9 terajoules
- 25.0 million kilowatt-hours (≈ 25 GW·h)
- 21.5 billion kilocalories (≈ 21 Tcal)
- 85.2 billion BTUs
or to the energy released by combustion of the following:
- 21.5 kilotons of TNT-equivalent energy (≈ 21 kt)
- 568,000 US gallons of automotive gasoline
Any time energy is generated, the process can be evaluated from an E = mc2 perspective.
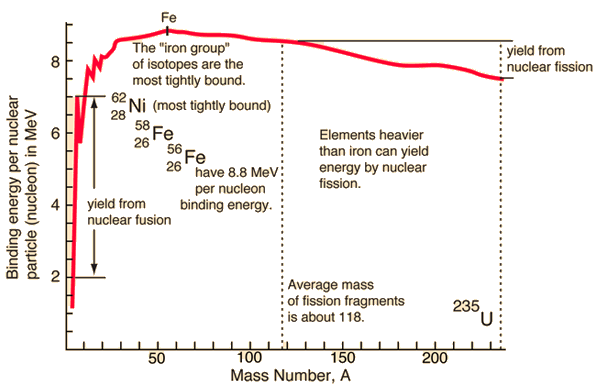
Nuclear Binding Energy – Mass Defect
Nuclear Energy and Electricity Production
Today we use the nuclear energy to generate useful heat and electricity. This electricity is generated in nuclear power plants. The heat source in the nuclear power plant is a nuclear reactor. As is typical in all conventional thermal power stations the heat is used to generate steam which drives a steam turbine connected to a generator which produces electricity. In 2011 nuclear power provided 10% of the world’s electricity. In 2007, the IAEA reported there were 439 nuclear power reactors in operation in the world, operating in 31 countries. They produce base-load electricity 24/7 without emitting any pollutants into the atmosphere (this includes CO2).
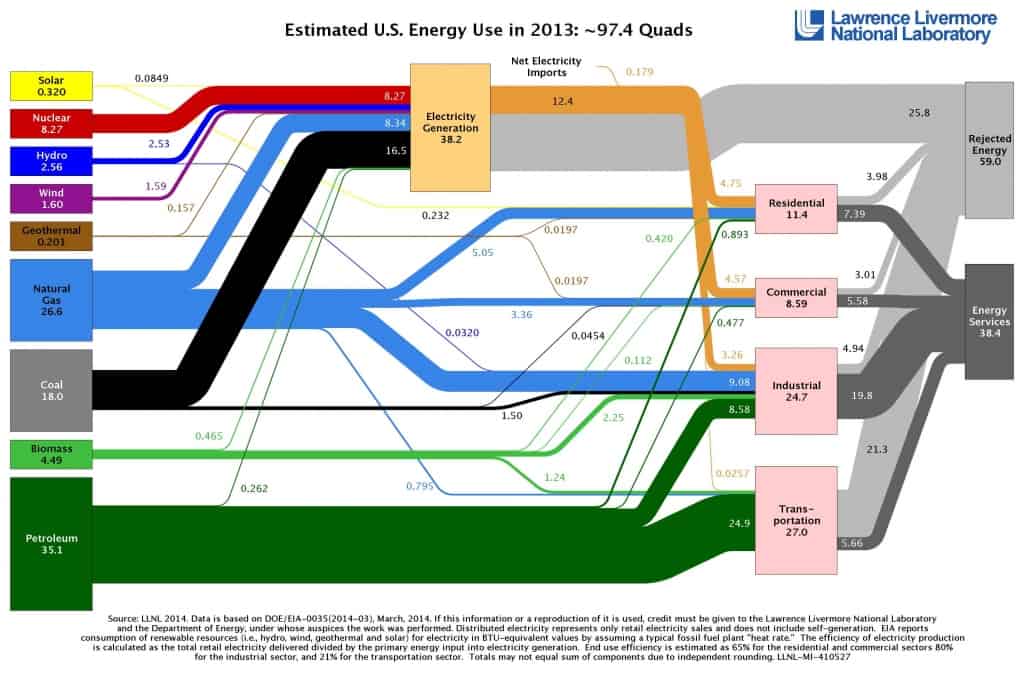
Nuclear Energy Consumption – Summary
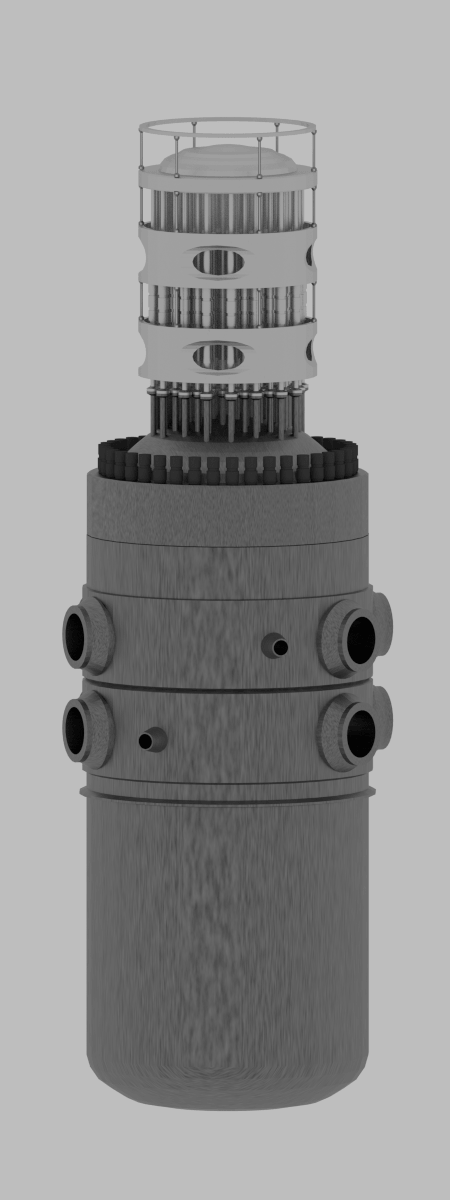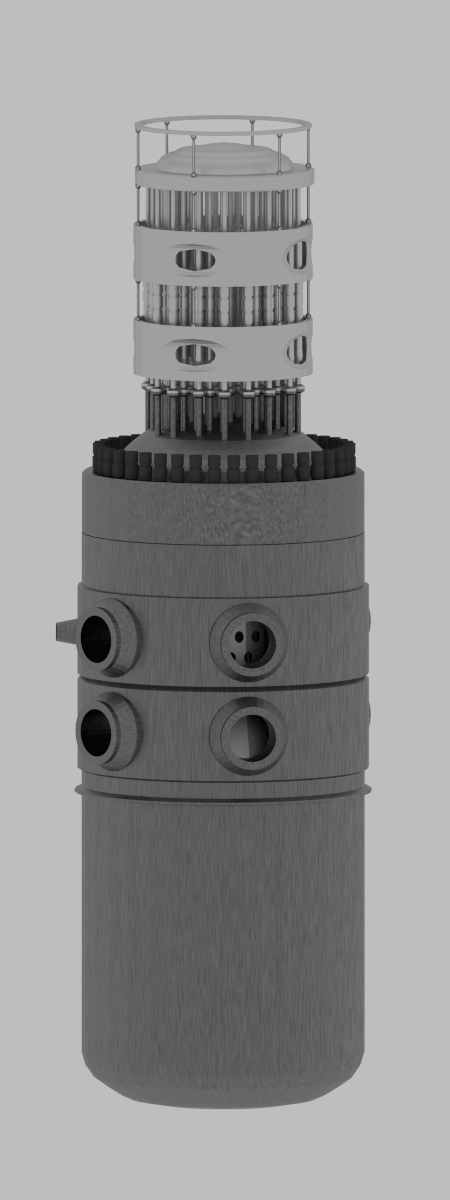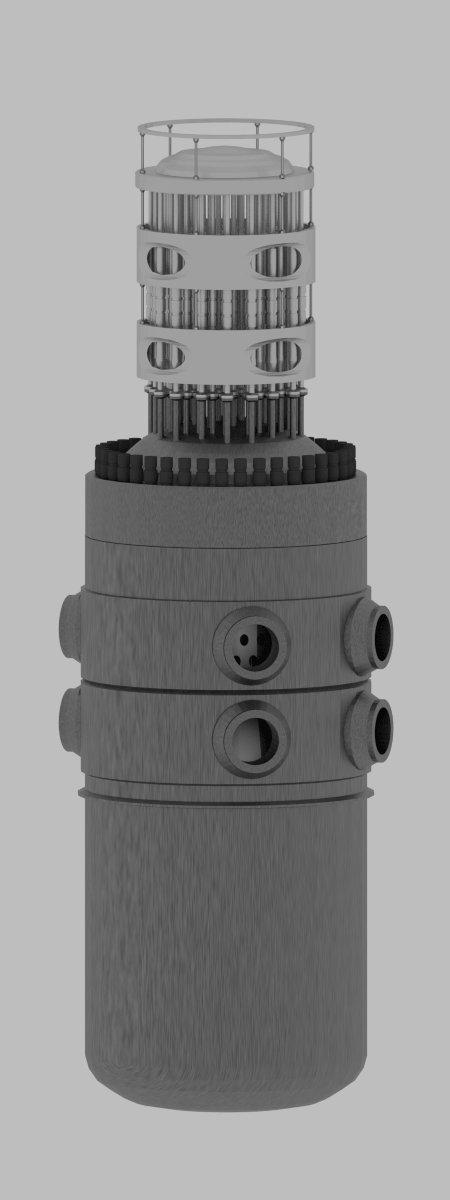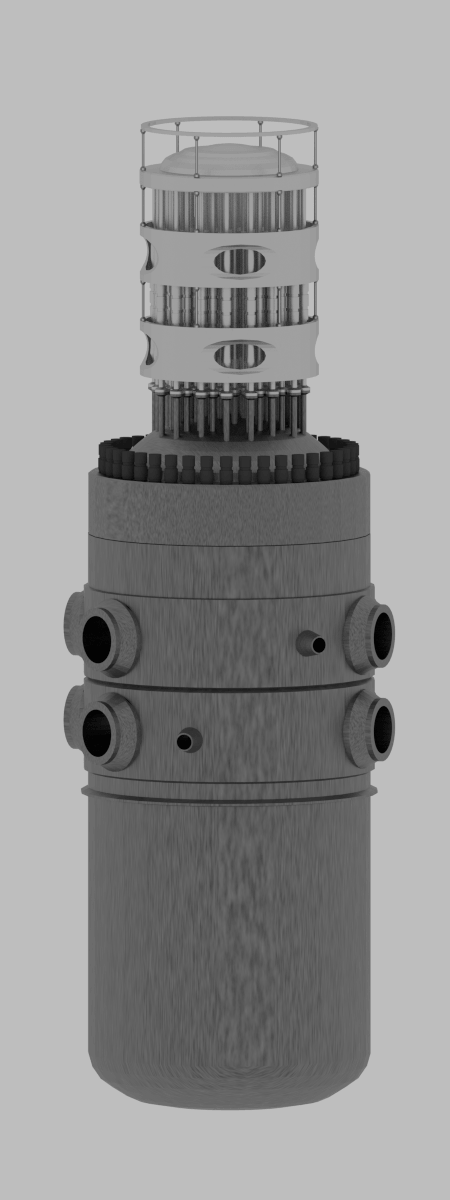
Consumption of a 3000MWth (~1000MWe) reactor (12-months fuel cycle)
It is an illustrative example, following data do not correspond to any reactor design.
- Typical reactor may contain about 165 tonnes of fuel (including structural material)
- Typical reactor may contain about 100 tonnes of enriched uranium (i.e. about 113 tonnes of uranium dioxide).
- This fuel is loaded within, for example, 157 fuel assemblies composed of over 45,000 fuel rods.
- A common fuel assembly contain energy for approximately 4 years of operation at full power.
- Therefore about one quarter of the core is yearly removed to spent fuel pool (i.e. about 40 fuel assemblies), while the remainder is rearranged to a location in the core better suited to its remaining level of enrichment (see Power Distribution).
- The removed fuel (spent nuclear fuel) still contains about 96% of reusable material (it must be removed due to decreasing kinf of an assembly).
- Annual natural uranium consumption of this reactor is about 250 tonnes of natural uranium (to produce of about 25 tonnes of enriched uranium).
- Annual enriched uranium consumption of this reactor is about 25 tonnes of enriched uranium.
- Annual fissile material consumption of this reactor is about 1 005 kg.
- Annual matter consumption of this reactor is about 1.051 kg.
- But it corresponds to about 3 200 000 tons of coal burned in coal-fired power plant per year.
See also: Fuel Consumption
We hope, this article, Nuclear Energy, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.