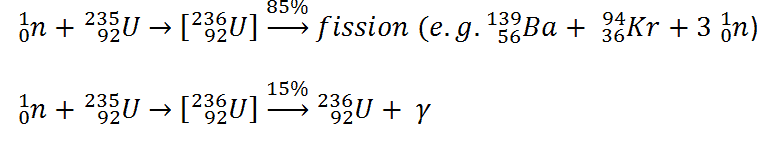Uranium 235
Uranium 235, which alone constitutes 0.72% of natural uranium is the second common isotope of uranium in the nature. This isotope has half-life of 7.04×108 years (6.5 times shorter than the isotope 238) and therefore its abundance is lower than 238U (99.28%). 235U belongs to primordial nuclides, because its half-life is comparable to the age of the Earth (~4.5×109 years). For its very long half-life, it is still present in the Earth’s crust. 235U decays via alpha decay (by way of thorium-231) into 231Pa. 235U occasionally decays by spontaneous fission with very low probability of 0.0000000072%.
235U is a fissile isotope, which means 235U is capable of undergoing fission reaction after absorbing thermal neutron. Moreover, 235U meets also alternative requirement that the amount (~2.43 per one fission by thermal neutron) of neutrons produced by fission of 235U is sufficient to sustain a nuclear fission chain reaction. 235U was the first isotope that was found to be fissile. In fact, 235U is the only existing fissile nucleus from naturally-occurring isotopes and therefore is a highly strategic material. At the time of the formation of the Earth, 235U was 85 times more abundant. The 0.72% observed today are only a residue caused by the difference in the half-lifes of 235U and 238U. If mankind had been present at the beginning of the Earth, they would not have needed to enrich uranium, because the content of fissile 235U was significantly higher.
Uranium 235 Fission
Uranium 235 is a fissile isotope and its fission cross-section for thermal neutrons is about 585 barns (for 0.0253 eV neutron). For fast neutrons its fission cross-section is on the order of barns. Most of absorption reactions result in fission reaction, but a minority results in radiative capture forming 236U. The cross-section for radiative capture for thermal neutrons is about 99 barns (for 0.0253 eV neutron). Therefore about 15% of all absorption reactions result in radiative capture of neutron. About 85% of all absorption reactions result in fission.
Typically, when uranium 235 nucleus undergoes fission, the nucleus splits into two smaller nuclei (triple fission can also rarely occur), along with a few neutrons (the average is 2.43 neutrons per fission by thermal neutron) and release of energy in the form of heat and gamma rays. The average of the fragment atomic mass is about 118, but very few fragments near that average are found. It is much more probable to break up into unequal fragments, and the most probable fragment masses are around mass 95 (Krypton) and 137 (Barium).
Most of the fission fragments are highly unstable (radioactive) nuclei and undergo further radioactive decays to stabilize itself. Therefore part of the released energy is radiated away from the reactor (See also: Reactor antineutrinos). On the other hand most of the energy released by one fission (~170MeV of total ~200MeV) appears as kinetic energy of these fission fragments. The fission fragments interact strongly with the surrounding atoms or molecules traveling at high speed, causing them to ionize. Creation of ion pairs requires energy, which is lost from the kinetic energy of the charged fission fragment causing it to decelerate. The positive ions and free electrons created by the passage of the charged fission fragment will then reunite, releasing energy in the form of heat (e.g. vibrational energy or rotational energy of atoms). This is the principle how fission fragments heat up fuel in the reactor core.
 Fission fragments after a nucleus fission. Fission fragments interact strongly with the surrounding atoms or molecules traveling at high speed, causing them to ionize.
Fission fragments after a nucleus fission. Fission fragments interact strongly with the surrounding atoms or molecules traveling at high speed, causing them to ionize.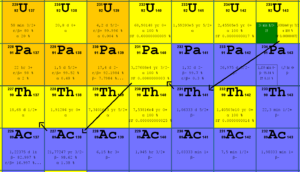 Uranium 235 decays via alpha decay into 231Pa.
Uranium 235 decays via alpha decay into 231Pa.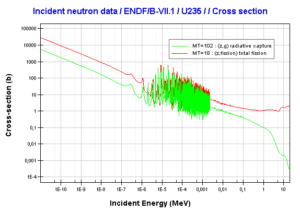 Uranium 235. Comparison of total fission cross-section and cross-section for radiative capture.
Uranium 235. Comparison of total fission cross-section and cross-section for radiative capture.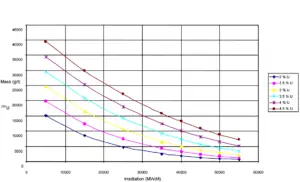 235U content as a function of burnup level of a PWR fuel.
235U content as a function of burnup level of a PWR fuel.Source: IAEA-TECDOC-1529 Management of
Reprocessed Uranium
 Neutron production per one fast fission of uranium 235.
Neutron production per one fast fission of uranium 235.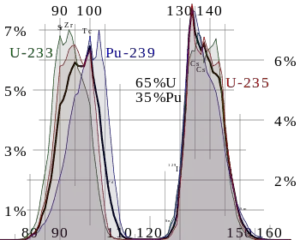 Fission fragment yield for different nuclei. The most probable fragment masses are around mass 95 (Krypton) and 137 (Barium).
Fission fragment yield for different nuclei. The most probable fragment masses are around mass 95 (Krypton) and 137 (Barium).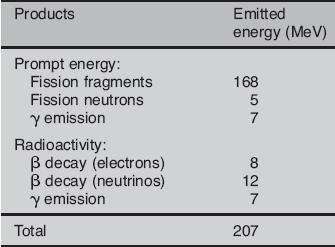
We hope, this article, Uranium 235, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.