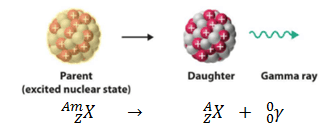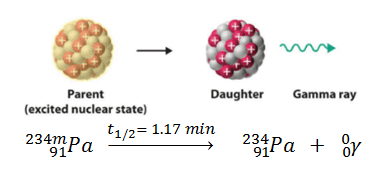Gamma decay or γ decay represents the disintegration of a parent nucleus to a daughter through the emission of gamma rays (high energy photons). This transition (γ decay) can be characterized as:
As can be seen, if a nucleus emits a gamma ray, atomic and mass numbers of daughter nucleus remain the same, but daughter nucleus will form different energy state of the same element. Note that, nuclides with equal proton number and equal mass number (thus making them by definition the same isotope), but in a different energy state are known as nuclear isomers. We usually indicate isomers with a superscript m, thus: 241mAm or 110mAg.
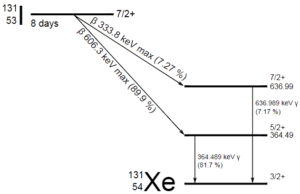
In most practical laboratory sources, the excited nuclear states are created in the decay of a parent radionuclide, therefore a gamma decay typically accompanies other forms of decay, such as alpha or beta decay. Typically after a beta decay (isobaric transition), nuclei usually contain too much energy to be in its final stable or daughter state.
Gamma rays are high-energy photons with very short wavelengths and thus very high frequency. Gamma rays from radioactive decay are in the energy range from a few keV to ~8 MeV, corresponding to the typical energy levels in nuclei with reasonably long lifetimes. As was written, they are produced by the decay of nuclei as they transition from a high energy state to a lower state. Since the gamma rays are in substance only a very high-energy photons, they are very penetrating matter and are thus biologically hazardous. Gamma rays can travel thousands of feet in air and can easily pass through the human body.
In contrast to alpha and beta radioactivity, gamma radioactivity is governed by an electromagnetic interaction rather than a weak or strong interaction. As in atomic transitions, the photon carries away at least one unit of angular momentum (the photon, being described by the vector electromagnetic field, has spin angular momentum of ħ), and the process conserves parity.
Prompt Gamma Decay
As was written, gamma decay may follow nuclear reactions such as neutron capture, nuclear fusion, or nuclear fission. Most of nuclear reactions produce extremely unstable nuclei that decay as soon as they are formed in nuclear reactions (half-life less than 10-11s) and are not generally classified as nuclear isomers. Moreover, these nuclei usually produce a cascade of gamma rays and the gamma-ray cascade concludes when all excess energy of the excited nucleus is released.
For example, following a nuclear fission prompt gamma rays are emitted from fission fragments. Most of prompt gamma rays are emitted after prompt neutrons. The fission reaction releases approximately ~7 MeV in prompt gamma rays and additional ~7 MeV (for 235U) in delayed gamma rays. This is a significant portion of energy (~7 % of fission energy released) and it must be considered in many fields of reactor design
Isomeric Transition
In certain cases, the excited nuclear state that follows the emission of a beta particle or other type of excitation, are able to stay in metastable state for a long time (hours, days and sometimes much longer) before undergoing gamma decay, in which they emit a gamma ray. These long-lived excited nuclei are known as isomeric states (or isomers) and their decays are termed isomeric transitions. The process of isomeric transition is therefore similar to any gamma emission, but differs in that it involves the intermediate metastable excited state(s) of the nuclei.
Metastable nuclei are often characterized by high nuclear spin, requiring a change in spin of several units or more with gamma decay, instead of a single unit transition that occurs in only 10−12 seconds. The rate of gamma decay is also slowed when the energy of excitation of the nucleus is small. An example is the decay of the isomer or metastable state of protactinium:
Extremely unstable nuclei that decay as soon as they are formed in nuclear reactions (half-life less than 10-11s) are not generally classified as nuclear isomers. Isomeric transitions must occur by higher order multipole transitions (in contrast to gamma emission that occurs by dipole radiation) that occur on a longer time-scale.
We hope, this article, Gamma Decay – Gamma Radioactivity, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.