Thorium 232
Thorium 232, which alone makes up nearly all natural thorium, is the most common isotope of thorium in the nature. This isotope has the longest half-life (1.4 x 1010 years) of all isotopes with more than 83 protons. In fact, its half-life is considerably longer than the age of earth. Therefore 232Th belongs to primordial nuclides.232Th decays via alpha decay into 228Ra . 232Th occasionally decays by spontaneous fission with very low probability of 1.1 x 10-9 %.
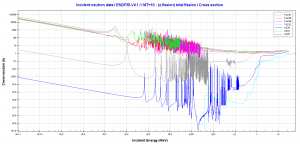
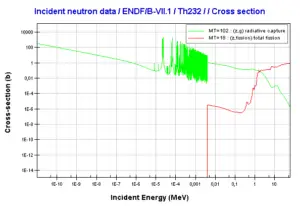
232Th is a fertile isotope. 232Th is not capable of undergoing fission reaction after absorbing thermal neutron, on the other hand 232Th can be fissioned by fast neutron with energy higher than >1MeV. 232Th does not meet also alternative requirement to fissile materials. 232Th is not capable of sustaining a nuclear fission chain reaction, because too many of neutrons produced by fission of 232Th have lower energies than original neutron.Isotope 232Th is key material in the thorium fuel cycle. Radiative capture of a neutron leads to the formation of fissile 233U. This process is called nuclear fuel breeding.
We hope, this article, Thorium 232, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.