Atomic and nuclear physics are not the same. The term atomic physics is often associated with nuclear power, due to the synonymous use of atomic and nuclear in standard English. However, physicists distinguish between atomic and nuclear physics. The atomic physics deals with the atom as a system consisting of a nucleus and electrons. The nuclear physics deals with the nucleus as a system consisting of a nucleons (protons and neutrons). Main difference is in the scale. While the term atomic deals with 1Å = 10-10m, where Å is an ångström (according to Anders Jonas Ångström), the term nuclear deals with 1femtometre = 1fermi = 10-15m.
Atomic Physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and the processes by which these arrangements change. This includes ions as well as neutral atoms and, unless otherwise stated, for the purposes of this discussion it should be assumed that the term atom includes ions. Atomic physics also help to understand the physics of molecules, but there is also molecular physics, which describes physical properties of molecules.
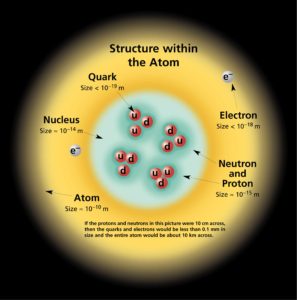
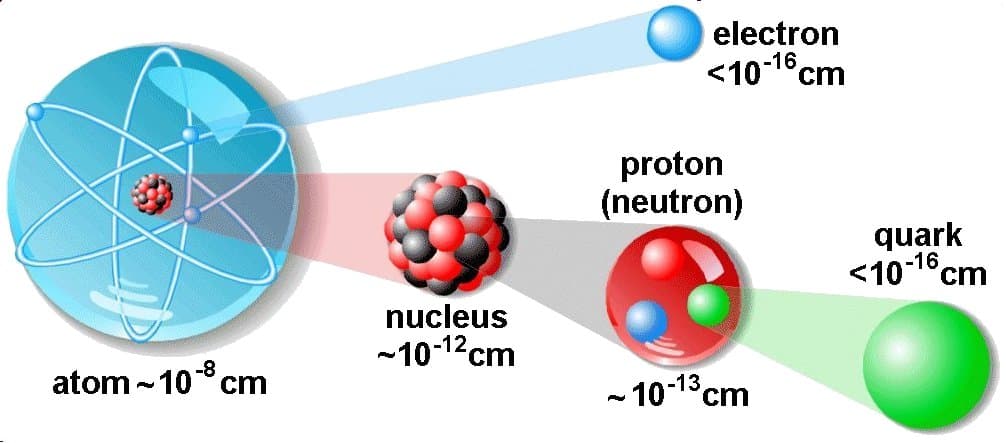
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Typical nuclear radii are of the order 10−14 m. Assuming spherical shape, nuclear radii can be calculated according to following formula:
r = r0 . A1/3
where r0 = 1.2 x 10-15 m = 1.2 fm
If we use this approximation, we therefore expect the volume of the nucleus to be of the order of 4/3πr3 or 7,23 ×10−45 m3 for hydrogen nuclei or 1721×10−45 m3 for 238U nuclei. These are volumes of nuclei and atomic nuclei (protons and neutrons) contains of about 99.95% of mass of atom.
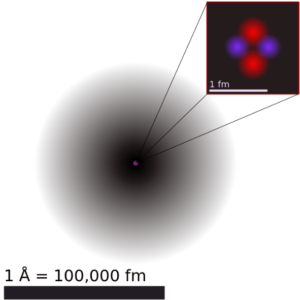
The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. For uranium atom, the Van der Waals radius is about 186 pm = 1.86 ×10−10 m. The Van der Waals radius, rw, of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom. Assuming spherical shape, the uranium atom have volume of about 26.9 ×10−30 m3. But this “huge” space is occupied primarily by electrons, because the nucleus occupies only about 1721×10−45 m3 of space. These electrons together weigh only a fraction (let say 0.05%) of entire atom.
It may seem, that the space and in fact the matter is empty, but it is not. Due to the quantum nature of electrons, the electrons are not point particles, they are smeared out over the whole atom. The classical description cannot be used to describe things on the atomic scale. On the atomic scale, physicists have found that quantum mechanics describes things very well on that scale. Particle locations in quantum mechanics are not at an exact position, they are described by a probability density function. Therefore the space in an atom (between electrons and an atomic nucleus) is not empty, but it is filled by a probability density function of electrons (usually known as “electron cloud“).
Nuclear Physics
Nuclear physics is the field of physics that studies the constituents (protons and neutrons) and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation, but the modern nuclear physics contains also particle physics, which is taught in close association with nuclear physics. The nuclear physics has provided application in many fields, including those in nuclear medicine (Positron Emission Tomography, isotopes production, etc.) and magnetic resonance imaging, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology.
These physical fundamentals consist of following topics:
See also: Fundamental Particles
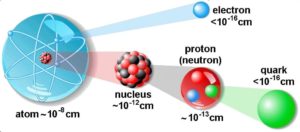
The physical world is composed of combinations of various subatomic or fundamental particles. These are the smallest building blocks of matter. All matter except dark matter is made of molecules, which are themselves made of atoms. The atoms consist of two parts. An atomic nucleus and an electron cloud. The electrons are spinning around the atomic nucleus. The nucleus itself is generally made of protons and neutrons but even these are composite objects. Inside the protons and neutrons, we find the quarks.
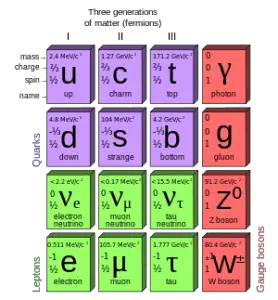
Quarks and electrons are some of the elementary particles. A number of fundamental particles have been discovered in various experiments. So many, that researchers had to organize them, just like Mendeleev did with his periodic table. This is summarized in a theoretical model (concerning the electromagnetic, weak, and strong nuclear interactions) called the Standard Model. In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions and the fundamental bosons. The fermions are generally “matter particles” and “antimatter particles”.
- Quarks. The quarks combine to form composite particles called hadrons, the best known and most stable are protons and neutrons
- Antiquarks. For every quark there is a corresponding type of antiparticle. The antiquarks have the same mass, mean lifetime, and spin as their respective quarks, but the electric charge and other charges have the opposite sign.
- Leptons. The best known of all leptons are the electrons and the neutrinos.
- Antileptons. For every lepton there is a corresponding type of antiparticle. The best known of all antileptons are the positrons and the antineutrinos.
The bosons are generally “force particles” that mediate interactions among fermions.
- Gauge bosons. The gauge boson is a force carrier of the fundamental interactions of nature.
- Higgs boson. The Higgs bosons give other particles mass via the Higgs mechanism. Their existence was confirmed by CERN on 14 March 2013.
 However, only a few of these fundamental particles (in fact, some of these are not fundamental particles) are very important in nuclear engineering. Nuclear engineering or theory of nuclear reactors operates with much better known subatomic particles such as:
However, only a few of these fundamental particles (in fact, some of these are not fundamental particles) are very important in nuclear engineering. Nuclear engineering or theory of nuclear reactors operates with much better known subatomic particles such as:
- Electrons. The electrons are negatively charged, almost massless particles that nevertheless account for most of the size of the atom. Electrons were discovered by Sir John Joseph Thomson in 1897. Electrons are located in an electron cloud, which is the area surrounding the nucleus of the atom. The electron is only one member of a class of elementary particles, which forms an atom.
- Protons. The protons are positively charged, massive particles that are located inside the atomic nucleus. Protons were discovered by Ernest Rutherford in the year 1919, when he performed his gold foil experiment.
- Neutron. Neutrons are located in the nucleus with the protons. Along with protons, they make up almost all of the mass of the atom. Neutrons were discovered by James Chadwick in 1932, when he demonstrated that penetrating radiation incorporated beams of neutral particles.
- Photon. A photon is an elementary particle, the force carrier for the electromagnetic force. The photon is the quantum of light (discrete bundle of electromagnetic energy). Photons are always in motion and, in a vacuum, have a constant speed of light to all observers (c = 2.998 x 108 m/s).
- Neutrino. A neutrino is an elementary particle, one of particles which make up the universe. Neutrinos are electrically neutral, weakly interacting and therefore able to pass through great distances in matter without being affected by it.
- Positron. Positron is an antiparticle of a negative electron. Positrons, also called positive electron, have a positive electric charge and have the same mass and magnitude of charge as the electron. An annihilation occurs, when a low-energy positron collides with a low-energy electron.
See also: Fundamental Particles
Source: chemwiki.ucdavis.edu
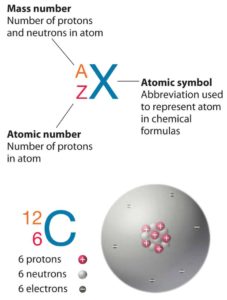
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. In a neutral atom there are as many electrons as protons moving about nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements.
Hydrogen (H), for example , consist of one electron and one proton. The number of neutrons in a nucleus is known as the neutron number and is given the symbol N. The total number of nucleons, that is, protons and neutrons in a nucleus, is equal to Z + N = A, where A is called the atomic mass number. The various species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides. Each nuclide is denoted by chemical symbol of the element (this specifies Z) with tha atomic mass number as supescript.
Thus the symbol 1H refers to the nuclide of hydrogen with a single proton as nucleus. 2H is the hydrogen nuclide with a neutron as well as a proton in the nucleus (2H is also called deuterium or heavy hydrogen). Atoms such as 1H, 2H whose nuclei contain the same number of protons but different number of neutrons (different A) are known as isotopes. Uranium, for instance, has three isotopes occuring in nature – 238U, 235U and 234U. The stable isotopes (plus a few of the unstable isotopes) are the atoms that are found in the naturally occuring elements in nature. However, they are not found in equal amounts. Some isotopes of a given element are more abundant than others. For example 99,27% of naturally occuring uranium atoms are the isotope 238U, 0,72% are the isotope 235U and 0,0055% are the isotope 234U. Exact structure of atoms is described by Atomic Theory and Theory of Nuclear Structure.
- Atomic Theory. Atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. The word atom comes from the Ancient Greek adjective atomos, meaning “uncuttable”. Today it is known that also atoms are divisible. Atomic Theory consist of many models and discoveries, which gradually formed this theory.
- Theory of Nuclear Structure. Understanding the structure of the atomic nucleus is one of the central challenges in modern nuclear physics.
See also: Atomic and Nuclear Structure
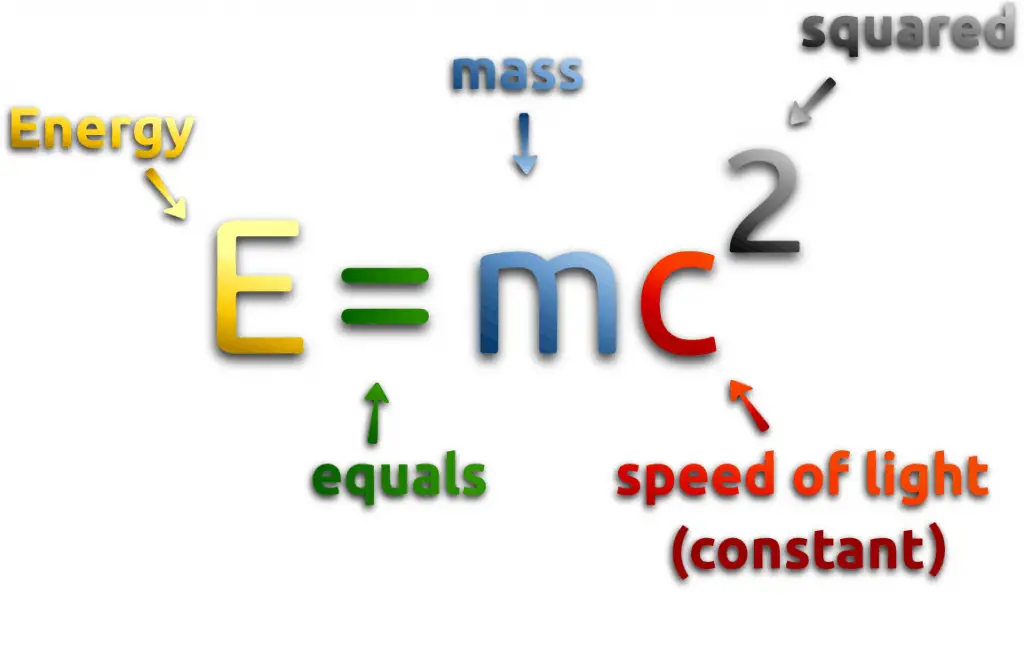
, where M is the small amount of mass and C is the speed of light.
What that means? If the nuclear energy is generated (splitting atoms, nuclear fussion), a small amount of mass transforms into the pure energy (such as kinetic energy, thermal energy, or radiant energy).
Example:
The energy equivalent of one gram (1/1000 of a kilogram) of mass is equivalent to:
89.9 terajoules
25.0 million kilowatt-hours (≈ 25 GW·h)
21.5 billion kilocalories (≈ 21 Tcal)
85.2 billion BTUs
or to the energy released by combustion of the following:
21.5 kilotons of TNT-equivalent energy (≈ 21 kt)
568,000 US gallons of automotive gasoline
Any time energy is generated, the process can be evaluated from an E = mc2 perspective.
Today we use the nuclear energy to generate useful heat and electricity. In 2011 nuclear power provided 10% of the world’s electricity. In 2007, the IAEA reported there were 439 nuclear power reactors in operation in the world, operating in 31 countries. They produce base-load electricity 24/7 without emitting any pollutants into the atmosphere (this includes CO2).
See also: Mass and Energy
Radiation
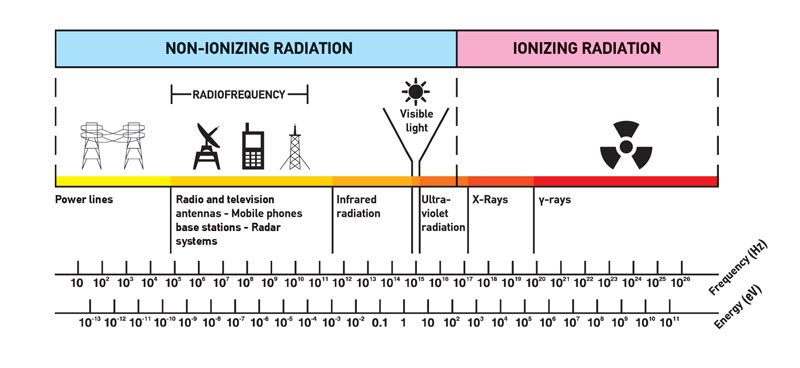
Most general definition is that radiation is energy that comes from a source and travels through some material or through space. Light, heat and sound are types of radiation. This is very general definition, the kind of radiation discussed in this article is called ionizing radiation. Most people connect the term radiation only with ionizing radiation, but it is not correct. Radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us. It is a part of our natural world that has been here since the birth of our planet. We should distinguish between:
- Non-ionizing radiation. The kinetic energy of particles (photons, electrons, etc.) of non-ionizing radiation is too small to produce charged ions when passing through matter. The particles (photons) have only sufficient energy to change the rotational, vibrational or electronic valence configurations of target molecules and atoms. Sunlight, radio waves, and cell phone signals are examples of non-ionizing (photon) radiation. However, it can still cause harm, like when you get a sunburn.
- Ionizing radiation. The kinetic energy of particles (photons, electrons, etc.) of ionizing radiation is sufficient and the particle can ionize (to form ion by losing electrons) target atoms to form ions. Simply ionizing radiation can knock electrons from an atom.
The boundary is not sharply defined, since different molecules and atoms ionize at different energies. This is typical for electromagnetic waves. Among electromagnetic waves belong, in order of increasing frequency (energy) and decreasing wavelength: radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays and gamma rays. Gamma rays, X-rays, and the higher ultraviolet part of the spectrum are ionizing, whereas the lower ultraviolet, visible light (including laser light), infrared, microwaves, and radio waves are considered non-ionizing radiation.
Forms of ionizing radiation
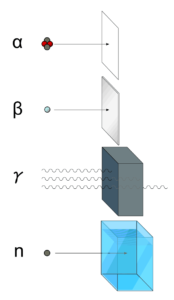
Ionizing radiation is categorized by the nature of the particles or electromagnetic waves that create the ionizing effect. These particles/waves have different ionization mechanisms, and may be grouped as:
- Directly ionizing. Charged particles (atomic nuclei, electrons, positrons, protons, muons, etc.) can ionize atoms directly by fundamental interaction through the Coulomb force if it carries sufficient kinetic energy. These particles must be moving at relativistic speeds to reach the required kinetic energy. Even photons (gamma rays and X-rays) can ionize atoms directly (despite they are electrically neutral) through the Photoelectric effect and the Compton effect, but secondary (indirect) ionization is much more significant.
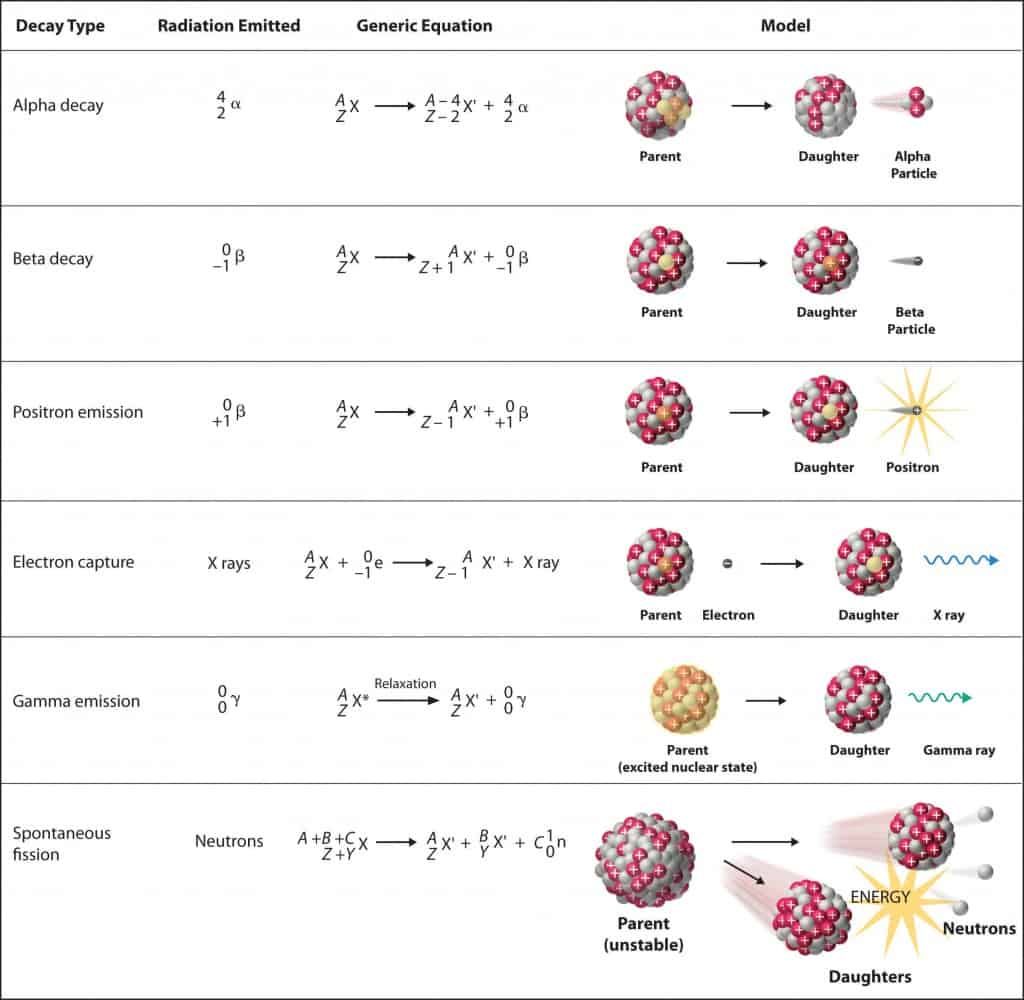
- Alpha radiations. Alpha radiation consist of alpha particles at high energy/speed. The production of alpha particles is termed alpha decay. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particles are relatively large and carry a double positive charge. They are not very penetrating and a piece of paper can stop them. They travel only a few centimeters but deposit all their energies along their short paths.
- Beta radiation. Beta radiation consist of free electrons or positrons at relativistic speeds. Beta particles (electrons) are much smaller than alpha particles. They carry a single negative charge. They are more penetrating than alpha particles, but thin aluminum metal can stop them. They can travel several meters but deposit less energy at any one point along their paths than alpha particles.
- Indirectly ionizing. Indirect ionizing radiation is electrically neutral particles and therefore does not interact strongly with matter. The bulk of the ionization effects are due to secondary ionizations.
- Photon radiation (Gamma or X-rays). Photon radiation consist of high energy photons. These photons are particles/waves (Wave-Particle Duality) without rest mass or electrical charge. They can travel 10 meters or more in air. This is a long distance compared to alpha or beta particles. However, gamma rays deposit less energy along their paths. Lead, water, and concrete stop gamma radiation. Photons (gamma rays and X-rays) can ionize atoms directly through the Photoelectric effect and the Compton effect, where the relatively energetic electron is produced. The secondary electron will go on to produce multiple ionization events, therefore the secondary (indirect) ionization is much more significant.
- Neutron radiation. Neutron radiation consist of free neutrons at any energies/speeds. Neutrons can be emitted by nuclear fission or by the decay of some radioactive atoms. Neutrons have zero electrical charge and cannot directly cause ionization. Neutrons ionize matter only indirectly. For example, when neutrons strike the hydrogen nuclei, proton radiation (fast protons) results. Neutrons can range from high speed, high energy particles to low speed, low energy particles (called thermal neutrons). Neutrons can travel hundreds of feet in air without any interaction.
See also: Radiation
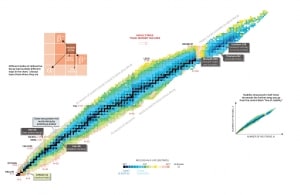 Nuclear Stability is a concept that helps to identify the stability of an isotope. To identify the stability of an isotope it is needed to find the ratio of neutrons to protons. To determine the stability of an isotope you can use the ratio neutron/proton (N/Z). Also to help understand this concept there is a chart of the nuclides, known as a Segre chart. This chart shows a plot of the known nuclides as a function of their atomic and neutron numbers. It can be observed from the chart that there are more neutrons than protons in nuclides with Z greater than about 20 (Calcium). These extra neutrons are necessary for stability of the heavier nuclei. The excess neutrons act somewhat like nuclear glue.
Nuclear Stability is a concept that helps to identify the stability of an isotope. To identify the stability of an isotope it is needed to find the ratio of neutrons to protons. To determine the stability of an isotope you can use the ratio neutron/proton (N/Z). Also to help understand this concept there is a chart of the nuclides, known as a Segre chart. This chart shows a plot of the known nuclides as a function of their atomic and neutron numbers. It can be observed from the chart that there are more neutrons than protons in nuclides with Z greater than about 20 (Calcium). These extra neutrons are necessary for stability of the heavier nuclei. The excess neutrons act somewhat like nuclear glue.
See also: Livechart – iaea.org

Source: Livechart – IAEA.org
Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge. These two forces compete, leading to various stability of nuclei. There are only certain combinations of neutrons and protons, which forms stable nuclei.
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus. If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay. Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, or electron capture. Many other rare types of decay, such as spontaneous fission or neutron emission are known. It should be noted that all of these decay pathways may be accompanied by the subsequent emission of gamma radiation. Pure alpha or beta decays are very rare.
Example:
Nuclei, such as 15O, which are lacking in neutrons (consist of 8 protons and 7 neutrons) undergo positron decay (positive beta decay). In this process, one of the protons in the nucleus is transformed into a neutron, positron and neutrino.The positron and the neutrino are emitted. The number of protons is thus reduced from 8 to 7 (number of neutrons is increased from 7 to 8), so that the resulting nucleus is an isotope of nitrogen, 15N, which is stable.
On the other hand nuclei, such as 19O, which have excess of neutrons, decay by negative beta decay, emitting a negative electron and an antineutrino. In this process, one of the neutrons in the nucleus is transformed into a proton. The number of protons is thus increased from 8 to 9 (number of neutrons is reduced from 11 to 10), so that the resulting nucleus is an isotope of fluor, 19F, which is stable. It should be noted that in both positive or negative beta decays the atomic mass number remains the same.
Of the first 82 elements in the periodic table, 80 have isotopes considered to be stable. Technetium, promethium and all the elements with an atomic number over 82 are unstable and decompose through radioactive decay. No undiscovered heavy elements (with atomic number over 110) are expected to be stable, therefore lead is considered the heaviest stable element. For each of the 80 stable elements, the number of the stable isotopes is given. For example, tin has 10 such stable isotopes.
There are 80 elements with at least one stable isotope, but 114 to 118 chemical elements are known. All elements to element 98 are found in nature, and the remainder of the discovered elements are artificially produced, with isotopes all known to be highly radioactive with relatively short half-lives.
Bismuth, thorium, uranium and plutonium are primordial nuclides because they have half-lives long enough to still be found on the Earth, while all the others are produced either by radioactive decay or are synthesized in laboratories and nuclear reactors. Primordial nuclides are nuclides found on the Earth that have existed in their current form since before Earth was formed. Primordial nuclides are residues from the Big Bang, from cosmogenic sources, and from ancient supernova explosions which occurred before the formation of the solar system. Only 288 such nuclides are known.
Connection between Nuclear Stability and Radioactive Decay
The nuclei of radioisotopes are unstable. In an attempt to reach a more stable arrangement of its neutrons and protons, the unstable nucleus will spontaneously decay to form a different nucleus. If the number of neutrons changes in the process (number of protons remains), a different isotopes is formed and an element remains (e.g. neutron emission). If the number of protons changes (different atomic number) in the process, then an atom of a different element is formed. This decomposition of the nucleus is referred to as radioactive decay. During radioactive decay an unstable nucleus spontaneosly and randomly decomposes to form a different nucleus (or a different energy state – gamma decay), giving off radiation in the form of atomic partices or high energy rays. This decay occurs at a constant, predictable rate that is referred to as half-life. A stable nucleus will not undergo this kind of decay and is thus non-radioactive.
See also: Nuclear Stability

Source: chemwiki.ucdavis.edu
Nuclear decay (Radioactive decay) occurs when an unstable atom loses energy by emitting ionizing radiation. Radioactive decay is a random process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. There are many types of radioactive decay:
- Alpha radioactivity. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Because of its very large mass (more than 7000 times the mass of the beta particle) and its charge, it heavy ionizes material and has a very short range.
- Beta radioactivity. Consist of beta particles. Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles have greater range of penetration than alpha particles, but still much less than gamma rays.The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay.
- Gamma radioactivity. Gamma radioactivity consist of gamma rays. Gamma rays are electromagnetic radiation (high energy photons) of an very high frequency and of a high energy. They are produced by the decay of nuclei as they transition from a high energy state to a lower state known as gamma decay. Most of nuclear reactions are accompanied by gamma emission.
- Neutron emission. Neutron emission is a type of radioactive decay of nuclei containing excess neutrons (especially fission products), in which a neutron is simply ejected from the nucleus. This type of radiation plays key role in nuclear reactor control, because these neutrons are delayed neutrons.
Radioactive decay law
Calculations of the decay of radioactive nuclei are relatively straightforward, owing to the fact that there is only one fundamental law governing all decay process. This law states that the probability per unit time that a nucleus will decay is a constant, independent of time. This constant is called the decay constant and is denoted by λ, “lambda”. The radioactive decay of certain number of atoms (mass) is exponential in time.
Radioactive decay law: N = N.e-λt
The rate of nuclear decay is also measured in terms of half-lives. The half-life is the amount of time it takes for a given isotope to lose half of its radioactivity. If a radioisotope has a half-life of 14 days, half of its atoms will have decayed within 14 days. In 14 more days, half of that remaining half will decay, and so on. Half lives range from millionths of a second for highly radioactive fission products to billions of years for long-lived materials (such as naturally occurring uranium). Notice that short half lives go with large decay constants. Radioactive material with a short half life is much more radioactive (at the time of production) but will obviously lose its radioactivity rapidly. No matter how long or short the half life is, after seven half lives have passed, there is less than 1 percent of the initial activity remaining.
The radioactive decay law can be derived also for activity calculations or mass of radioactive material calculations:
(Number of nuclei) N = N.e-λt (Activity) A = A.e-λt (Mass) m = m.e-λt
, where N (number of particles) is the total number of particles in the sample, A (total activity) is the number of decays per unit time of a radioactive sample, m is the mass of remaining radioactive material.
See also: Radioactive Decay
Nuclear reactors are devices to initiate and control a chain nuclear reaction, but there are not only manmade devices. The world’s first nuclear reactor operated about two billion years ago. The natural nuclear reactor formed at Oklo in Gabon, Africa, when a uranium-rich mineral deposit became flooded with groundwater that acted as a neutron moderator, and a nuclear chain reaction started. These fission reactions were sustained for hundreds of thousands of years, until a chain reaction could no longer be supported. This was confirmed by existence of isotopes of the fission-product gas xenon and by different ratio of U-235/U238 (enrichment of natural uranium).
Notation of nuclear reactions
Standard nuclear notation shows (see picture) the chemical symbol, the mass number and the atomic number of the isotope.
If the initial nuclei are denoted by a and b, and the product nuclei are denoted by c and d, the reaction can be represented by the equation:
a + b → c + d
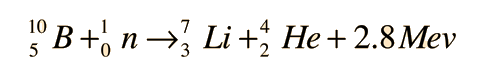

Source: chemwiki.ucdavis.edu
Instead of using the full equations in the style above, in many situations a compact notation is used to describe nuclear reactions. This style of the form a(b,c)d is equivalent to a + b producing c + d. Light particles are often abbreviated in this shorthand, typically p means proton, n means neutron, d means deuteron, α means an alpha particle or helium-4, β means beta particle or electron, γ means gamma photon, etc. The reaction above would be written as 10B(n,α)7Li.
Nuclear reactions
Although the number of possible nuclear reactions is enormous, nuclear reactions can be sorted by types. Most of nuclear reactions are accompanied by gamma emission. Some examples are:
- Elastic scattering. Occurs, when no energy is transferred between the target nucleus and the incident particle.
208Pb (n, n) 208Pb
- Inelastic scattering. Occurs, when energy is transferred. The difference of kinetic energies is saved in excited nuclide.
40Ca (α, α’) 40mCa
- Capture reactions. Both charged and neutral particles can be captured by nuclei. This is accompanied by the emission of ˠ-rays. Neutron capture reaction produces radioactive nuclides (induced radioactivity).
238U (n, ˠ) 239U
- Rearrangement Reactions. The absorption of a particle accompanied by the emission of one or more particles is called a rearrangement reaction.
4He (α, p) 7Li
- Fission reactions. Nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a large amount of energy.
235U (n, 3 n) fission products
- Fusion reactions. Occur when, two or more atomic nuclei collide at a very high speed and join to form a new type of atomic nucleus.The fusion reaction of deuterium and tritium is particularly interesting because of its potential of providing energy for the future.
3T (d, n) 4He
- Spallation reactions. Occur, when a nucleus is hit by a particle with sufficient energy and momentum to knock out several small fragments or, smash it into many fragments.
- Nuclear decay (Radioactive decay). Occurs when an unstable atom loses energy by emitting ionizing radiation. Radioactive decay is a random process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. There are many types of radioactive decay:
- Alpha radioactivity. Alha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Because of its very large mass (more than 7000 times the mass of the beta particle) and its charge, it heavy ionizes material and has a very short range.
- Beta radioactivity. Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles have greater range of penetration than alpha particles, but still much less than gamma rays.The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay.
- Gamma radioactivity. Gamma rays are electromagnetic radiation of an very high frequency and are therefore high energy photons. They are produced by the decay of nuclei as they transition from a high energy state to a lower state known as gamma decay. Most of nuclear reactions are accompanied by gamma emission.
- Neutron emission. Neutron emission is a type of radioactive decay of nuclei containing excess neutrons (especially fission products), in which a neutron is simply ejected from the nucleus. This type of radiation plays key role in nuclear reactor control, because these neutrons are delayed neutrons.
Source: chemwiki.ucdavis.edu
Fundamental laws
For purposes of this article it is sufficient to note four of the fundamental laws governing these reactions.
- Conservation of nucleons. The total number of nucleons before and after a reaction are the same.
- Conservation of charge. The sum of the charges on all the particles before and after a reaction are the same
- Conservation of momentum. The total momentum of the interacting particles before and after a reaction are the same.
- Conservation of energy. Energy, including rest mass energy, is conserved in nuclear reactions.
Reference: Lamarsh, John R. Introduction to Nuclear engineering 2nd Edition.
See also: Nuclear Reactions
Source: hyperphysics.phy-astr.gsu.edu
A binding energy is generally the energy required to disassemble a whole system into separate parts. It is known the sum of separate parts has typically a higher potential energy than a bound system, therefore the bound system is more stable. A creation of bound system is often accompanied by subsequent energy release. We usually distinguish the binding energy according to these levels:
At atomic level the atomic binding energy of the atom derives from electromagnetic interaction of electrons in the atomic cloud and nucleons (protons) in the nucleus. The atomic binding energy is the energy required to disassemble an atom into free electrons and a nucleus. This is more commonly known as ionization energy.
At molecular level the molecular binding energy of the molecule derives from bond-dissociation energy of atoms in a chemical bond.
At nuclear level the nuclear binding energy is the energy required to disassemble (to overcome the strong nuclear force) a nucleus of an atom into its component parts (protons and neutrons).
Nuclear binding energy
The component parts of nuclei are neutrons and protons, which are collectively called nucleons. The mass of a nucleus is always less than the sum masses of the constituent protons and neutrons when separated. The difference is a measure of the nuclear binding energy which holds the nucleus together. According to the Einstein relationship (E=m.c2) this binding energy is proportional to this mass difference and it is known as the mass defect.
During the nuclear splitting or nuclear fusion, some of the mass of the nucleus gets converted into huge amounts of energy and thus this mass is removed from the total mass of the original particles, and the mass is missing in the resulting nucleus. The nuclear binding energies are enormous, they are on the order of a million times greater than the electron binding energies of atoms.
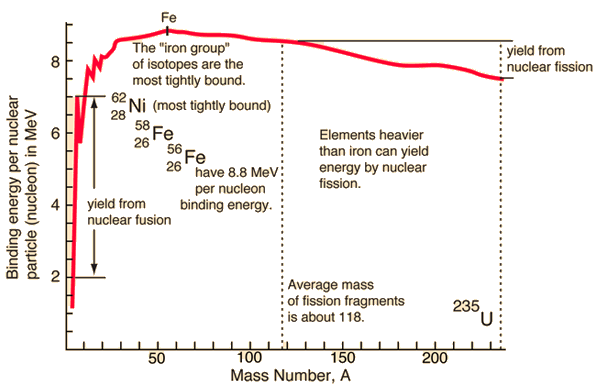
Nuclear Binding Curve
If the splitting releases energy and the fusion releases the energy, so where is the breaking point? For understanding this issue it is better to relate the binding energy to one nucleon, to obtain nuclear binding curve. The binding energy per one nucleon is not linear. There is a peak in the binding energy curve in the region of stability near iron and this means that either the breakup of heavier nuclei than iron or the combining of lighter nuclei than iron will yield energy.
The reason the trend reverses after iron peak is the growing positive charge of the nuclei. The electric force has greater range than strong nuclear force. While the strong nuclear force binds only close neighbors the electric force of each proton repels the other protons.
See also: Binding Energy
We hope, this article, Atomic and Nuclear Physics, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.