Atomic Theory
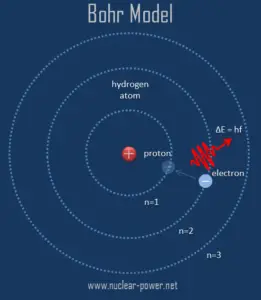 As was written, all matter except dark matter is made of molecules, which are themselves made of atoms. The atoms are the smallest constituents of ordinary matter, which can be divided without the release of electrically charged particles. The atoms consist of two parts. An atomic nucleus and an electron cloud. The electrons are spinning around the atomic nucleus. The nucleus itself is generally made of protons and neutrons.
As was written, all matter except dark matter is made of molecules, which are themselves made of atoms. The atoms are the smallest constituents of ordinary matter, which can be divided without the release of electrically charged particles. The atoms consist of two parts. An atomic nucleus and an electron cloud. The electrons are spinning around the atomic nucleus. The nucleus itself is generally made of protons and neutrons.
This model is today quite clear and proven. But the path to this model was complex and full of many surprising findings. A scientific theory, which deals with the nature of matter is known as atomic theory. Atomic theory began as a philosophical concept in ancient Greece and India. It must be added, atomism was one of a number of competing theories on the nature of matter. These ancient philosophers speculated that the earth was made up of different combinations of basic substances, or elements.
The word “atom” was coined by the ancient Greek philosophers Leucippus, Epicurus and Democritus. They supposed that the properties of materials were determined by the different shapes and forms of their atoms. This word comes from the Ancient Greek adjective atomos, meaning “indivisible“.
Other ancient Greek philosophers (Empedocles, Heraclitus and Aristotle) considered these basic elements to be earth, air, water, and fire. According to their philosophy, lead differs from gold only in the proportions of the these four elements it contains and so the problem of converting lead into gold is just a matter of correctly blending the right combination of elements. Unlike the atomism of Democritus and Epicurus, the Aristotelian “natural minimum” was not conceptualized as physically indivisible.
Modem ideas about the structure of matter originated in the 17th century. In 1661 the English chemist Robert Boyle laid down the modern criterion of an element published the modern criterion for an element:
“a basic substance that cannot be broken down into any simpler substance after it is isolated from a compound, but can be combined with other elements to form compounds”
To date, 105 different elements have been confirmed to exist. Of the 105 confirmed elements, 90 exist in nature and 15 are man-made.
We hope, this article, Atomic Theory, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.