Discovery of Proton
Before the discovery of atomic nucleus, there was ideas that all atoms are composed of hydrogen atoms (called by William Prout “protyles“). This hypothesis is known as Prout’s hypothesis. In 1816, he hypothesized that the hydrogen atom was the only truly fundamental particles, and that the other atoms were actually groupings of various numbers of hydrogen atoms (protyles). Further important facts were discovered in 1886 by German physicist Eugen Goldstein. He discovered anode rays and showed that they were positively charged particles (ions) produced from gases. In 1907 a study of how these rays were deflected in a magnetic field, revealed that the particles making up the ray were not all the same mass. The lightest ones, formed when there was some hydrogen gas in the tube, were calculated to be about 1840 times as massive as an electron.
Following Geiger-Marsden experiments, which were a landmark series of experiments by which scientists discovered that every atom contains an atomic nucleus (whose diameter is of the order 10-14m) lead to the identification of the ‘primitive atom’ . The identification of the primitive atom of positive electricity was first made by the British physicist Ernest Rutherford. Rutherford proved that the hydrogen nucleus is present in other nuclei, a result usually described as the discovery of protons. No positively charged atom lighter than these had ever been detected. Furthermore, they never carry more than a single natural unit of positive charge, +e.
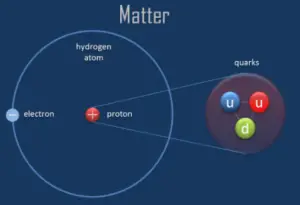 According to modern physics, a proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter. It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10−27 kg (938.272 MeV/c2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. The proton has a mean square radius of about 0.87 × 10−15 m, or 0.87 fm, and it is a spin – ½ fermion.
According to modern physics, a proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter. It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10−27 kg (938.272 MeV/c2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. The proton has a mean square radius of about 0.87 × 10−15 m, or 0.87 fm, and it is a spin – ½ fermion.
The protons exist in the nuclei of typical atoms, along with their neutral counterparts, the neutrons. Neutrons and protons, commonly called nucleons, are bound together in the atomic nucleus, where they account for 99.9 percent of the atom’s mass. Research in high-energy particle physics in the 20th century revealed that neither the neutron nor the proton is not the smallest building block of matter.
We hope, this article, Discovery of Proton, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.