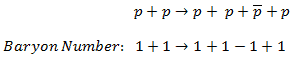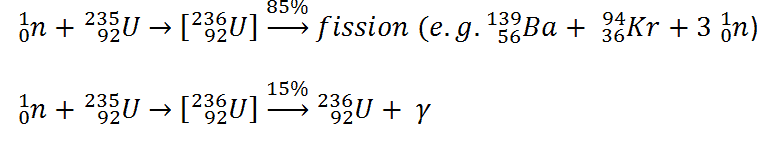Atomic Mass Number – Does it conserve in a nuclear reaction?
 In general, the atomic mass number is not conserved in nuclear reactions.
In general, the atomic mass number is not conserved in nuclear reactions.
In analyzing nuclear reactions, we apply the many conservation laws. Nuclear reactions are subject to classical conservation laws for charge, momentum, angular momentum, and energy (including rest energies). Additional conservation laws, not anticipated by classical physics, are are electric charge, lepton number and baryon number. Certain of these laws are obeyed under all circumstances, others are not. We have accepted conservation of energy and momentum. In reactor physics (non-relativistic physics), we assume that the number of protons (the atomic number), the number of neutrons (the neutron number) and its sum (the atomic mass number) are usually separately conserved. We shall find circumstances and conditions in which this rule is not true. Where we are considering non-relativistic nuclear reactions, it is essentially true. However, where we are considering relativistic nuclear energies or those involving the weak interactions (e.g. in beta decay the atomic number is not conserved), we shall find that these principles must be extended.
Instead of mass number conservation, physicists define the baryon number, which is a conserved quantum number in all particle reactions.
Baryon number is a generalization of nucleon number, which is conserved in nonrelativistic nuclear reactions and decays. The law of conservation of baryon numberstates that:
The sum of the baryon number of all incoming particles is the same as the sum of the baryon numbers of all particles resulting from the reaction.
For example, the following reaction (proton-antiproton pair production) does conserve B and does occur if the incoming proton has sufficient energy (the threshold energy = 5.6 GeV):
As indicated, B = +2 on both sides of this equation.
From these and other reactions, the conservation of baryon number has been established as a basic principle of physics.
This principle provides basis for the stability of the proton. Since the proton is the lightest particle among all baryons, the hypothetical products of its decay would have to be non-baryons. Thus, the decay would violate the conservation of baryon number. It must be added some theories have suggested that protons are in fact unstable with very long half-life (~1030 years) and that they decay into leptons. There is currently no experimental evidence that proton decay occurs.
Neutron Number – Does it conserve in a nuclear reaction?
In general, the neutron number is not conserved in nuclear reactions.
Atomic Number – Does it conserve in a nuclear reaction?
In general, the atomic number is not conserved in nuclear reactions.
Instead of atomic number conservation, an universal conservation law is the conservation of the electric charge.
Conservation of Electric Charge
As was written, the total electrical charge of the nucleus is determined by the atomic number and is equal to +Ze. Conservation of charge is thought to be a universal conservation law. No experimental evidence for any violation of this principle has ever been observed.
For example:
Consider a typical fission reaction such as the one listed below.
Typically, when uranium 235 nucleus undergoes fission, the nucleus splits into two smaller nuclei (triple fission can also rarely occur), along with a few neutrons (the average is 2.43 neutrons per fission by thermal neutron) and release of energy in the form of heat and gamma rays. From these reactions we find that in the fission the parent nucleus:
- 235U contains 92 protons (a charge of +92e),
- incident neutron is electrically neutral.
The fission fragments:
- 139Ba contains 56 protons (a charge of +56e),
- 94Ba contains 36 protons (a charge of +36e)
We see that the total charge is 92e before and after the reaction; thus, charge is conserved. It is noteworthy, the total number of nucleons before and after a reaction are also the same.
We hope, this article, Conservation of Atomic Number, Neutron Number and Mass Number, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.