Source: dev.physicslab.org

Source: imgkid.com
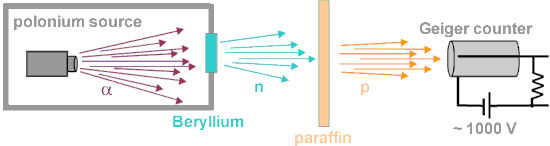
An experimental breakthrough came in 1930 with the observation by Bothe and Becker. They found that if the very energetic alpha particles emitted from polonium fell on certain light elements, specifically beryllium, boron, or lithium, an unusually penetrating radiation was produced. Since this radiation was not influenced by an electric field (neutrons have no charge), they presumed it was gamma rays (but much more penetrating). It was shown (Curie and Joliot) that when a paraffin target with this radiation is bombarded, it ejected protons with energy about 5.3 MeV. Paraffin is high in hydrogen content, hence offers a target dense with protons (since neutrons and protons have almost equal mass, protons scatter energetically from neutrons).These experimental results were difficult to interpret. James Chadwick was able to prove that the neutral particle could not be a photon by bombarding targets other than hydrogen, including nitrogen, oxygen, helium and argon. Not only were these inconsistent with photon emission on energy grounds, the cross-section for the interactions was orders of magnitude greater than that for Compton scattering by photons. In Rome, the young physicist Ettore Majorana suggested that the manner in which the new radiation interacted with protons required a new neutral particle.
The task was that of determining the mass of this neutral particle. James Chadwick chose to bombard boron with alpha particles and analyze the interaction of the neutral particles with nitrogen. These particlular targets were chosen partly because the masses of boron and nitrogen were well known. Using kinematics, Chadwick was able to determine the velocity of the protons. Then through conservation of momentum techniques, he was able to determine that the mass of the neutral radiation was almost exactly the same as that of a proton. In 1932, Chadwick proposed that the neutral particle was Rutherford’s neutron. In 1935, he was awarded the Nobel Prize for his discovery.
We hope, this article, Discovery of the Neutron, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.