Enthalpy in Chemical Reactions
The enthalpy is widely used also in chemistry. Chemical reactions are determined by the laws of thermodynamics. In thermodynamics, the internal energy of a system is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the potential energy of the system as a whole due to external force fields. The enthalpy of a chemical reaction is defined as the enthalpy change observed in a constituent of a thermodynamic system when one mole of substance reacts completely.
Since most of the chemical reactions in laboratory are constant-pressure processes, we can write the change in enthalpy (also known as enthalpy of reaction) for a reaction. The enthalpy of reaction can be positive or negative or zero depending upon whether the heat is gained or lost or no heat is lost or gained. In an endothermic reaction, the products have more stored chemical energy than the reactants. In an exothermic reaction, the opposite is true. The products have less stored chemical energy than the reactants. The excess energy is generally released to the surroundings when the reaction occurs.
In chemical reactions, energy is stored in the chemical bonds between the atoms that make up the molecules. Energy storage on the atomic level includes energy associated with electron orbital states. Whether a chemical reaction absorbs or releases energy, there is no overall change in the amount of energy during the reaction. That’s because of the law of conservation of energy, which states that:
Energy cannot be created or destroyed. Energy may change form during a chemical reaction.
Combustion of Hydrogen
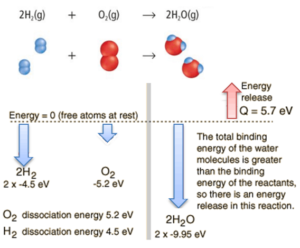
Consider the combustion of hydrogen in air. In a flame of pure hydrogen gas, burning in air, the hydrogen (H2) reacts with oxygen (O2) to form water (H2O) and releases energy.
Energetically, the process can be considered to require the energy to dissociate the H2 and O2, but then the bonding of the H2O returns the system to a bound state with negative potential. It is actually more negative than the bound states of the reactants, and the formation of the two water molecules is therefore an exothermic reaction, which releases 5.7 eV of energy. In words of enthalpy, the enthalpy of combustion is −286 kJ/mol:
2H2(g) + O2(g) → 2H2O(g)
In words of enthalpy, the enthalpy of combustion is −286 kJ/mol (energy per mol of molecular hydrogen):
2H2(g) + O2(g) → 2H2O(l) +572 kJ
The balance of energy before and after the reaction can be illustrated schematically with the state in which all atoms are free taken as the reference for energy.
We hope, this article, Enthalpy of Chemical Reaction, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.