Photoelectric Absorption of X-rays
Source: laradioactivite.com/
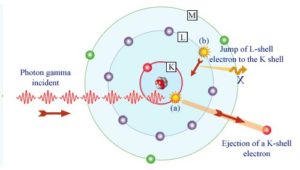
In the photoelectric effect, a photon undergoes an interaction with an electron which is bound in an atom. In this interaction the incident photon completely disappears and an energetic photoelectron is ejected by the atom from one of its bound shells. The kinetic energy of the ejected photoelectron (Ee) is equal to the incident photon energy (hν) minus the binding energy of the photoelectron in its original shell (Eb).
Ee=hν-Eb
Therefore photoelectrons are only emitted by the photoelectric effect if photon reaches or exceeds a threshold energy – the binding energy of the electron – the work function of the material. For very high X-rays with energies of more than hundreds keV, the photoelectron carries off the majority of the incident photon energy – hν.
At small values of gamma ray energy the photoelectric effect dominates. The mechanism is also enhaced for materials of high atomic number Z. It is not simple to derive analytic expression for the probability of photoelectric absorption of gamma ray per atom over all ranges of gamma ray energies. The probability of photoelectric absorption per unit mass is approximately proportional to:
τ(photoelectric) = constant x ZN/E3.5
where Z is the atomic number, the exponent n varies between 4 and 5. E is the energy of the incident photon. The proportionality to higher powers of the atomic number Z is the main reason for using of high Z materials, such as lead or depleted uranium in gamma ray shields.
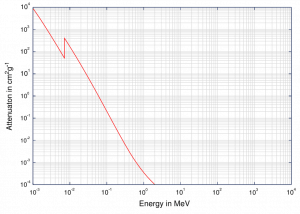 Although the probability of the photoelectric absorption of photon decreases, in general, with increasing photon energy, there are sharp discontinuities in the cross-section curve. These are called “absoption edges” and they correspond to the binding energies of electrons from atom’s bound shells. For photons with the energy just above the edge, the photon energy is just sufficient to undergo the photoelectric interaction with electron from bound shell, let say K-shell. The probability of such interaction is just above this edge much greater than that of photons of energy slightly below this edge. For photons below this edge the interaction with electron from K-shell in energetically impossible and therefore the probability drops abruptly. These edges occur also at binding energies of electrons from other shells (L, M, N …..).
Although the probability of the photoelectric absorption of photon decreases, in general, with increasing photon energy, there are sharp discontinuities in the cross-section curve. These are called “absoption edges” and they correspond to the binding energies of electrons from atom’s bound shells. For photons with the energy just above the edge, the photon energy is just sufficient to undergo the photoelectric interaction with electron from bound shell, let say K-shell. The probability of such interaction is just above this edge much greater than that of photons of energy slightly below this edge. For photons below this edge the interaction with electron from K-shell in energetically impossible and therefore the probability drops abruptly. These edges occur also at binding energies of electrons from other shells (L, M, N …..).
Interaction of X-rays with Matter
Although a large number of possible interactions are known, there are three key interaction mechanisms with matter. The strength of these interactions depends on the energy of the X-rays and the elemental composition of the material, but not much on chemical properties, since the X-ray photon energy is much higher than chemical binding energies. The photoelectric absorbtion dominates at low-energies of X-rays, while Compton scattering dominates at higher energies.
- Photoelectric absorption
- Compton scattering
- Rayleigh scattering
We hope, this article, Photoelectric Absorption of X-rays, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.