- Limiting Time. The amount of radiation exposure depends directly (linearly) on the time people spend near the source of radiation. The dose can be reduced by limiting exposure time.
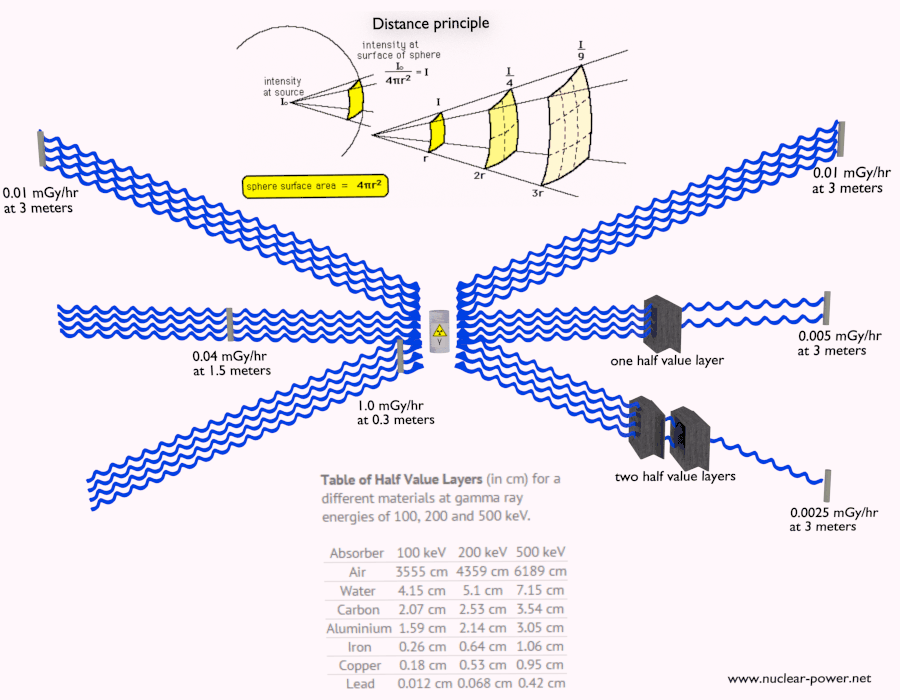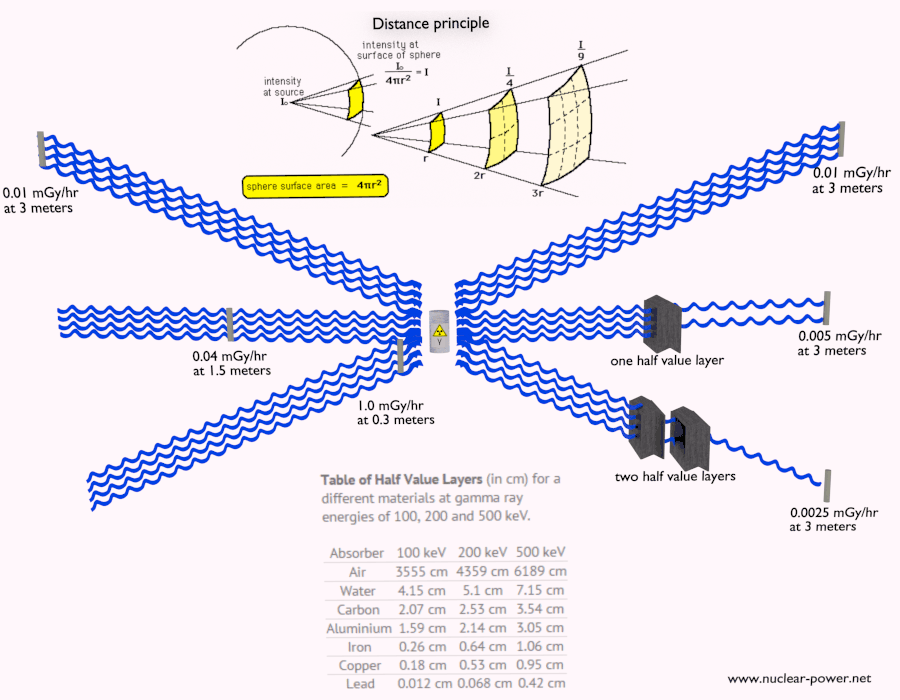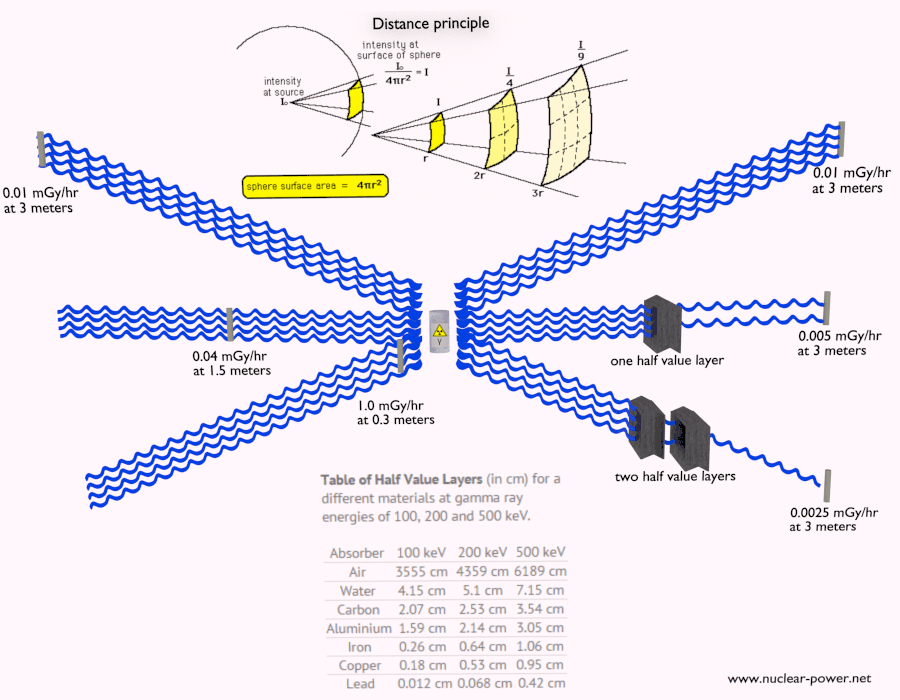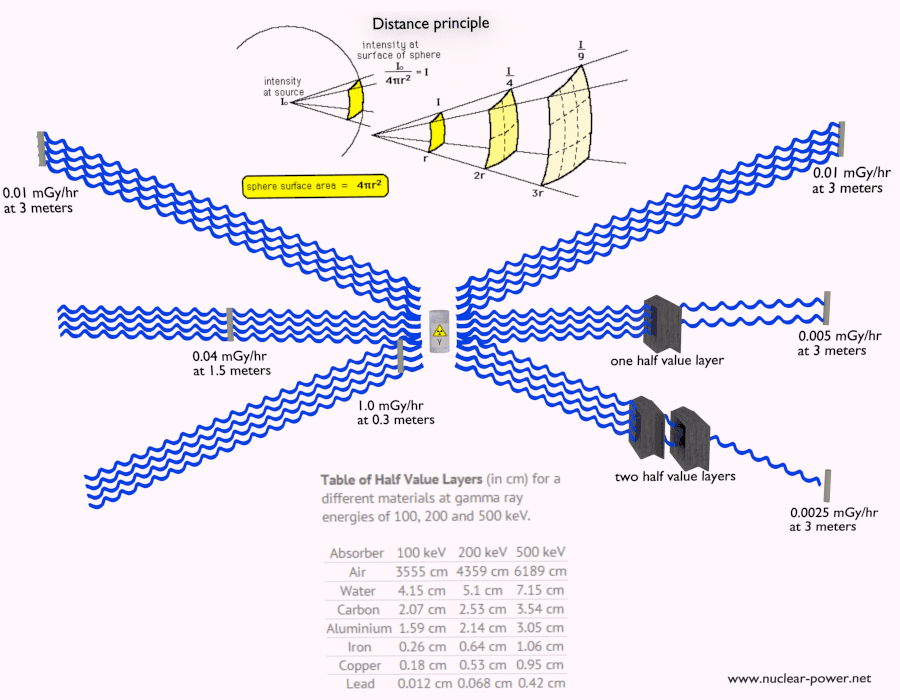
- Distance. The amount of radiation exposure depends on the distance from the source of radiation. Similarly to a heat from a fire, if you are too close, the intensity of heat radiation is high and you can get burned. If you are at the right distance, you can withstand there without any problems and moreover it is comfortable. If you are too far from heat source, the insufficiency of heat can also hurt you. This analogy, in a certain sense, can be applied to radiation also from nuclear sources.
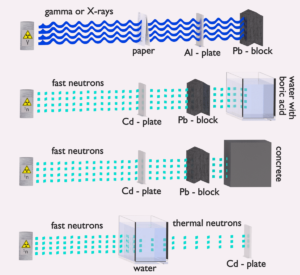
- Shielding. Finally, if the source is too intensive and time or distance do not provide sufficient radiation protection the shielding must be used. Radiation shielding usually consist of barriers of lead, concrete or water. Even depleted uranium can be used as a good protection from gamma radiation, but on the other hand uranium is absolutely inappropriate shielding of neutron radiation. In short, it depends on type of radiation to be shielded, which shielding will be effective or not.
Shielding of Neutrons
There are three main features of neutrons, which are crucial in the shielding of neutrons.
- Neutrons have no net electric charge, therefore they cannot be affected or stopped by electric forces. Neutrons ionize matter only indirectly, which makes neutrons highly penetrating type of radiation.
- Neutrons scatter with heavy nuclei very elastically. Heavy nuclei very hard slow down a neutron let alone absorb a fast neutron.
- An absorption of neutron (one would say shielding) causes initiation of certain nuclear reaction (e.g. radiative capture or even fission), which is accompanied by a number of other types of radiation. In short, neutrons make matter radioactive, therefore with neutrons we have to shield also the other types of radiation.
See also: Interaction of Neutrons with Matter
Principles of Neutron Shielding
The best materials for shielding neutrons must be able to:
- Slow down neutrons (the same principle as the neutron moderation). First point can be fulfilled only by material containing light atoms (e.g. hydrogen atoms), such as water, polyethylene, and concrete. The nucleus of a hydrogen nucleus contains only a proton. Since a proton and a neutron have almost identical masses, a neutron scattering on a hydrogen nucleus can give up a great amount of its energy (even entire kinetic energy of a neutron can be transferred to a proton after one collision). This is similar to a billiard. Since a cue ball and another billiard ball have identical masses, the cue ball hitting another ball can be made to stop and the other ball will start moving with the same velocity. On the other hand, if a ping pong ball is thrown against a bowling ball (neutron vs. heavy nucleus), the ping pong ball will bounce off with very little change in velocity, only a change in direction. Therefore lead is quite ineffective for blocking neutron radiation, as neutrons are uncharged and can simply pass through dense materials.
-
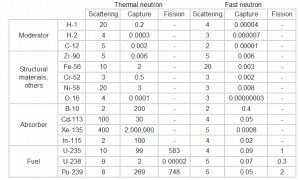
Table of cross-sections Absorb this slow neutron. Thermal neutrons can be easily absorbed by capture in materials with high neutron capture cross sections (thousands of barns) like boron, lithium or cadmium. Generally, only a thin layer of such absorbator is sufficient to shield thermal neutrons. Hydrogen (in the form of water), which can be used to slow down neutrons, have absorbtion cross-section 0.3 barns. This is not enough, but this insufficiency can be offset by sufficient thickness of water shield.
- Shield the accompanying radiation. In the case of cadmium shield the absorption of neutrons is accompanied by strong emission of gamma rays. Therefore additional shield is necessary to attenuate the gamma rays. This phenomenon practically does not exist for lithium and is much less important for boron as a neutron absorption material. For this reason, materials containing boron are used often in neutron shields. In addition, boron (in the form of boric acid) is well soluble in water making this combination very efective neutron shield.
Water as a neutron shield
Water due to the high hydrogen content and the availability is efective and common neutron shielding. However, due to the low atomic number of hydrogen and oxygen, water is not acceptable shield against the gamma rays. On the other hand in some cases this disadvantage (low density) can be compensated by high thickness of the water shield. In case of neutrons, water perfectly moderates neutrons, but with absorption of neutrons by hydrogen nucleus secondary gamma rays with the high energy are produced. These gamma rays highly penetrates matter and therefore it can increase requirements on the thickness of the water shield. Adding a boric acid can help with this problem (neutron absorbtion on boron nuclei without strong gamma emission), but results in another problems with corrosion of construction materials.
Concrete as a neutron shield
Most commonly used neutron shielding in many sectors of the nuclear science and engineering is shield of concrete. Concrete is also hydrogen-containing material, but unlike water concrete have higher density (suitable for secondary gamma shielding) and does not need any maintenance. Because concrete is a mixture of several different materials its composition is not constant. So when referring to concrete as a neutron shielding material, the material used in its composition should be told correctly. Generally concrete are divided to “ordinary “ concrete and “heavy” concrete. Heavy concrete uses heavy natural aggregates such as barites (barium sulfate) or magnetite or manufactured aggregates such as iron, steel balls, steel punch or other additives. As a result of these additives, heavy concrete have higher density than ordinary concrete (~2300 kg/m3). Very heavy concrete can achieve density up to 5,900 kg/m3 with iron additives or up to 8900 kg/m3 with lead additives. Heavy concrete provide very effective protection against neutrons.
We hope, this article, Shielding of Neutron Radiation, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.