Discovery of Electron
For almost 100 years (from Dalton’s A New System of Chemical Philosophy) atoms were thought to be the smallest possible division of matter. All of the results of chemical experiments during this time indicated that the atom was indivisible. Eventually, experimentation into electricity and radioactivity indicated that particles of matter smaller than the atom did indeed exist.
In 1897, an English physicist J.J. Thomson showed that cathode rays were composed of previously unknown negatively charged particles, which he calculated must have bodies much smaller than atoms and a very large value for their charge-to-mass ratio. He showed that these charged particles could be obtained from just about any material. For example, by the action of ultra-violet light on metals, by heating metal wires or by the ionising action of X-rays. Thomson originally called these particles “corpuscles,” and the name electron was proposed for these particles by the Irish physicist George Johnstone Stoney. It was evident that electrons are a component of all neutral atoms, the counterbalancing positive charge being carried by one or more of their other constituent parts. Thomson thus concluded that atoms are divisible, and that the corpuscles are their building blocks.
In 1906, J. J. Thompson won the Nobel Prize in physics for establishing the existence of electrons. According to current state of knowledge, the electrons are negatively charged (-1e), almost massless particles that nevertheless account for most of the size of the atom. Their rest mass equal to 9.109 × 10−31 kg (510.998 keV/c2) (approximately 1/1836 that of the proton). Electrons are located in an electron cloud, which is the area surrounding the nucleus of the atom.
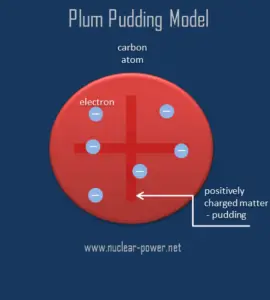
But the original idea was different. The original model of an atom, based on the discovery of the electron, was proposed by J. J. Thomson in 1904 and is known as the Plum pudding model or the Thomson model of the atom. It must be noted, this model was proposed before the discovery of the atomic nucleus.
The Plum pudding model represented an attempt to consolidate the known properties of atoms at the time:
1) Electrons are negatively-charged particles
2) Atoms are neutrally-charged.
According to this model, an atom consist of a sphere of positive matter within which electrostatic forces determined the positioning of the negatively charged corpuscles. This fact explains the overall neutral charge of the atom, Thomson proposed that the corpuscles were distributed in a uniform sea of positive charge, in which they are scattered like “the plums in a pudding”.
We hope, this article, Discovery of Electron, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.