Hydrogen
Helium
Lithium
92.8 nΩ⋅m
Beryllium
36 nΩ⋅m
Boron
1e15 nΩ⋅m
Carbon
7837 nΩ⋅m
Nitrogen
Oxygen
Fluorine
Neon
Sodium
47.7 nΩ⋅m
Magnesium
43.9 nΩ⋅m
Aluminium
26.5 nΩ⋅m
Silicon
2.3e12 nΩ⋅m
Phosphorus
Sulfur
2e24 nΩ⋅m
Chlorine
1e10 nΩ⋅m
Argon
Potassium
72 nΩ⋅m
Calcium
33.6 nΩ⋅m
Scandium
562 nΩ⋅m
Titanium
420 nΩ⋅m
Vanadium
197 nΩ⋅m
Chromium
125 nΩ⋅m
Manganese
1440 nΩ⋅m
Iron
96.1 nΩ⋅m
Cobalt
62.4 nΩ⋅m
Nickel
69.3 nΩ⋅m
Copper
16.8 nΩ⋅m
Zinc
59 nΩ⋅m
Gallium
270 nΩ⋅m
Germanium
1e9 nΩ⋅m
Arsenic
333 nΩ⋅m
Selenium
Bromine
8e19 nΩ⋅m
Krypton
Rubidium
128 nΩ⋅m
Strontium
132 nΩ⋅m
Yttrium
596 nΩ⋅m
Zirconium
421 nΩ⋅m
Niobium
152 nΩ⋅m
Molybdenum
53.4 nΩ⋅m
Technetium
200 nΩ⋅m
Ruthenium
71 nΩ⋅m
Rhodium
43.3 nΩ⋅m
Palladium
105 nΩ⋅m
Silver
15.9 nΩ⋅m
Cadmium
72.7 nΩ⋅m
Indium
83.7 nΩ⋅m
Tin
115 nΩ⋅m
Antimony
417 nΩ⋅m
Tellurium
Iodine
1e16 nΩ⋅m
Xenon
Caesium
205 nΩ⋅m
Lanthanoids
Hafnium
331 nΩ⋅m
Tantalum
131 nΩ⋅m
Tungsten
52.8 nΩ⋅m
Rhenium
193 nΩ⋅m
Osmium
81.2 nΩ⋅m
Iridium
47 nΩ⋅m
Platinum
105 nΩ⋅m
Gold
22.14 nΩ⋅m
Mercury
961 nΩ⋅m
Thallium
180 nΩ⋅m
Lead
208 nΩ⋅m
Bismuth
1290 nΩ⋅m
Polonium
400 nΩ⋅m
Astatine
Radon
Francium
Radium
1000 nΩ⋅m
Actinoids
Rutherfordium
Dubnium
Seaborgium
Bohrium
Hassium
Meitnerium
Darmstadtium
Roentgenium
Copernicium
Nihonium
Flerovium
Moscovium
Livermorium
Tennessine
Oganesson
Lanthanum
615 nΩ⋅m
Cerium
828 nΩ⋅m
Praseodymium
700 nΩ⋅m
Neodymium
643 nΩ⋅m
Promethium
750 nΩ⋅m
Samarium
940 nΩ⋅m
Europium
900 nΩ⋅m
Gadolinium
1310 nΩ⋅m
Terbium
1150 nΩ⋅m
Dysprosium
926 nΩ⋅m
Holmium
814 nΩ⋅m
Erbium
860 nΩ⋅m
Thulium
676 nΩ⋅m
Ytterbium
250 nΩ⋅m
Lutetium
582 nΩ⋅m
Actinium
Thorium
157 nΩ⋅m
Protactinium
177 nΩ⋅m
Uranium
280 nΩ⋅m
Neptunium
1220 nΩ⋅m
Plutonium
1460 nΩ⋅m
Americium
690 nΩ⋅m
Curium
1250 nΩ⋅m
Berkelium
Californium
Einsteinium
Fermium
Mendelevium
Nobelium
Lawrencium
Livermorium – Electrical Resistivity and Electrical Conductivity
Electrical resistivity of Livermorium is — nΩ·m.
Electrical resistivity and its converse, electrical conductivity, is a fundamental property of a material that quantifies how strongly it resists or conducts the flow of electric current. A low resistivity indicates a material that readily allows the flow of electric current. The symbol of resistivity is usually the Greek letter ρ (rho). The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). Note that, electrical resistivity is not the same as electrical resistance. Electrical resistance is expressed in Ohms. While resistivity is a material property, resistance is the property of an object.
Conductors – Semiconductors – Resistors
Substances in which electricity can flow are called conductors. Conductors are made of high-conductivity materials such as metals, in particular copper and aluminium.
Insulators, on the other hand, are made of a wide variety of materials depending on factors such as the desired resistance.
Semiconductors are materials, inorganic or organic, which have the ability to control their conduction depending on chemical structure, temperature, illumination, and presence of dopants. The name semiconductor comes from the fact that these materials have an electrical conductivity between that of a metal, like copper, gold, etc. and an insulator, such as glass. They have an energy gap less than 4eV (about 1eV). In solid-state physics, this energy gap or band gap is an energy range between valence band and conduction band where electron states are forbidden. In contrast to conductors, electrons in a semiconductor must obtain energy (e.g. from ionizing radiation) to cross the band gap and to reach the conduction band.
To understand the difference between metals, semiconductors and electrical insulators, we have to define the following terms from solid-state physics:
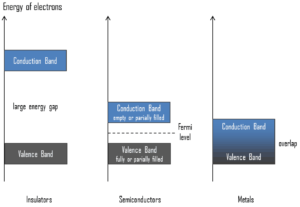 Valence Band. In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. In electrical insulators and semiconductors, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature. For example, a silicon atom has fourteen electrons. In the ground state, they are arranged in the electron configuration [Ne]3s23p2. Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands.
Valence Band. In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. In electrical insulators and semiconductors, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature. For example, a silicon atom has fourteen electrons. In the ground state, they are arranged in the electron configuration [Ne]3s23p2. Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands.- Conduction Band. In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. In electrical insulators and semiconductors, the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it. In semiconductors, electrons may reach the conduction band, when they are excited, for example, by ionizing radiation (i.e. they must obtain energy higher than Egap). For example, diamond is a wide-band gap semiconductor (Egap = 5.47 eV) with high potential as an electronic device material in many devices. On the other side, germanium has a small band gap energy (Egap = 0.67 eV), which requires to operate the detector at cryogenic temperatures. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands.
- Band Gap. In solid-state physics, the energy gap or the band gap is an energy range between valence band and conduction band where electron states are forbidden. In contrast to conductors, electrons in a semiconductor must obtain energy (e.g. from ionizing radiation) to cross the band gap and to reach the conduction band. Band gaps are naturally different for different materials. For example, diamond is a wide-band gap semiconductor (Egap = 5.47 eV) with high potential as an electronic device material in many devices. On the other side, germanium has a small band gap energy (Egap = 0.67 eV), which requires to operate the detector at cryogenic temperatures.
- Fermi Level. The term “Fermi level” comes from Fermi-Dirac statistics, which describes a distribution of particles over energy states in systems consisting of fermions (electrons) that obey the Pauli exclusion principle. Since they cannot exist in identical energy states, Fermi level is the term used to describe the top of the collection of electron energy levels at absolute zero temperature. The Fermi level is the surface of Fermi sea at absolute zero where no electrons will have enough energy to rise above the surface. In metals, the Fermi level lies in the hypothetical conduction band giving rise to free conduction electrons. In semiconductors the position of the Fermi level is within the band gap, approximately in the middle of the band gap.
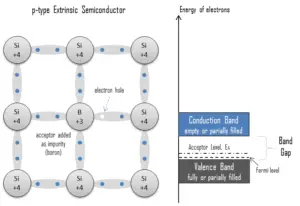 Electron-hole Pair. In the semiconductor, free charge carriers are electrons and electron holes(electron-hole pairs). Electrons and holes are created by excitation of electron from valence band to the conduction band. An electron hole (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or atomic lattice. It is one of the two types of charge carriers that are responsible for creating electric current in semiconducting materials. Since in a normal atom or crystal lattice the negative charge of the electrons is balanced by the positive charge of the atomic nuclei, the absence of an electron leaves a net positive charge at the hole’s location. Positively charged holes can move from atom to atom in semiconducting materials as electrons leave their positions. When an electron meets with a hole, they recombine and these free carriers effectively vanish. The recombination means an electron which has been excited from the valence band to the conduction band falls back to the empty state in the valence band, known as the holes.
Electron-hole Pair. In the semiconductor, free charge carriers are electrons and electron holes(electron-hole pairs). Electrons and holes are created by excitation of electron from valence band to the conduction band. An electron hole (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or atomic lattice. It is one of the two types of charge carriers that are responsible for creating electric current in semiconducting materials. Since in a normal atom or crystal lattice the negative charge of the electrons is balanced by the positive charge of the atomic nuclei, the absence of an electron leaves a net positive charge at the hole’s location. Positively charged holes can move from atom to atom in semiconducting materials as electrons leave their positions. When an electron meets with a hole, they recombine and these free carriers effectively vanish. The recombination means an electron which has been excited from the valence band to the conduction band falls back to the empty state in the valence band, known as the holes.
–