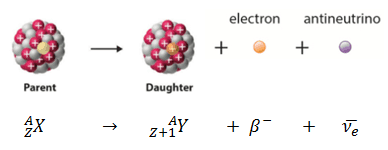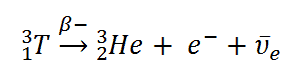Beta decay or β decay represents the disintegration of a parent nucleus to a daughter through the emission of the beta particle. This transition (β– decay) can be characterized as:
If a nucleus emits a beta particle, it loses an electron (or positron). In this case, the mass number of daughter nucleus remains the same, but daughter nucleus will form different element.
Example of Beta Decay
Free Neutron
A free neutron is a neutron that is not bounded in a nucleus. The free neutron is, unlike a bounded neutron, subject to radioactive beta decay.
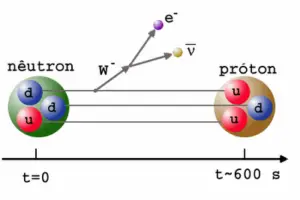
Source: scienceblogs.com
It decays into a proton, an electron, and an antineutrino (the antimatter counterpart of the neutrino, a particle with no charge and little or no mass). A free neutron will decay with a half-life of about 611 seconds (10.3 minutes). This decay involves the weak interaction and is associated with a quark transformation (a down quark is converted to an up quark). The decay of the neutron is a good example of the observations which led to the discovery of the neutrino. Because it decays in this manner, the neutron does not exist in nature in its free state, except among other highly energetic particles in cosmic rays. Since free neutrons are electrically neutral, they pass through the electrical fields within atoms without any interaction and they are interacting with matter almost exclusively through relatively rare collisions with atomic nuclei.
It must be noted, a proton cannot decay in free space. That is, a proton can undergo decay, but only inside a nucleus. It is due to the conservation of baryon number that has been established as a basic principle of physics. This principle provides basis for the stability of the proton. Since the proton is the lightest particle among all baryons, the hypothetical products of its decay would have to be non-baryons.
See also: Stability of Proton
Discovery of Neutrino
The study of beta decay provided the first physical evidence for the existence of the neutrino. The discovery of the neutrino is based on the law of conservation of energy during the process of beta decay.
See also: Discovery of Neutrino
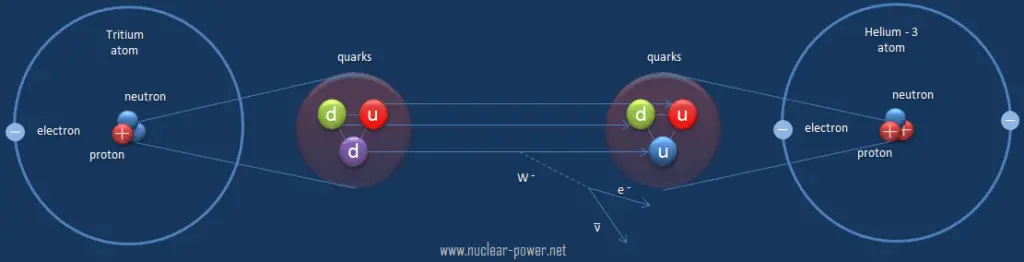
Beta Decay of Tritium
Tritium is a radioactive isotope, bur it emits a very weak form of radiation, a low-energy beta particle that is similar to an electron. It is a pure beta emitter (i.e. beta emitter without an accompanying gamma radiation). The electron’s kinetic energy varies, with an average of 5.7 keV, while the remaining energy is carried off by the nearly undetectable electron antineutrino. Such a very low energy of electron causes, that the electron cannot penetrate the skin or even does not travel very far in air. Beta particles from tritium can penetrate only about 6.0 mm of air.
Tritium decays via negative beta decay into helium-3 with half-life of 12.3 years.
We hope, this article, Example of Beta Decay, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.