There are the following forms of beta decay:
- Negative Beta Decay – Electron Decay. In electron decay, a neutron-rich nucleus emits a high-energy electron (β– particle). The electrons are negatively charged almost massless particles Due to the law of conservation of electric charge, the nuclear charge must increase by one unit. In this case, the process can be represented by:
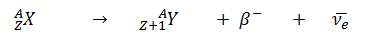
- Positive Beta Decay – Positron Decay. In positron decay, a proton-rich nucleus emits a positron (positrons are antiparticles of electrons, and have the same mass as electrons but positive electric charge), and thereby reduces the nuclear charge by one unit. In this case, the process can be represented by: An annihilation occurs, when a low-energy positron collides with a low-energy electron.
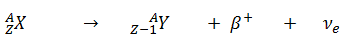
- Inverse Beta Decay – Electron Capture. Electron capture, known also as inverse beta decay is sometimes included as a type of beta decay, because the basic nuclear process, mediated by the weak interaction, is the same. In this process, a proton-rich nucleus can also reduce its nuclear charge by one unit by absorbing an atomic electron.
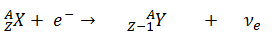
The emission of electrons was among the earliest observed decay phenomena. The inverse process, electron capture, was first observed by Luis Alvarez, in vanadium 48. He reported it in a 1937 paper in Physical Review.
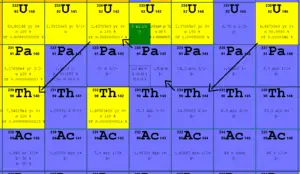
In a nuclear reactor occurs especially the β− decay, because the common feature of the fission products is an excess of neutrons (see Nuclear Stability). An unstable fission fragment with the excess of neutrons undergoes β− decay, where the neutron is converted into a proton, an electron, and an electron antineutrino. A free neutron also undergo this type of decay. A free neutron will decay with a half-life of about 611 seconds (10.3 minutes) into a proton, an electron, and an antineutrino (the antimatter counterpart of the neutrino, a particle with no charge and little or no mass).
We hope, this article, Type of Beta Decay, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.