Oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. The chemical symbol for Oxygen is O.
Neutron Number and Mass Number of Oxygen
Mass numbers of typical isotopes of Oxygen are 16; 17; 18.
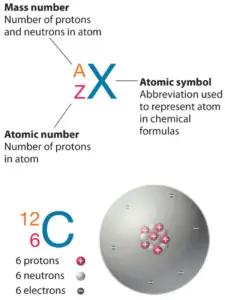 The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.
The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.
Neutron number is rarely written explicitly in nuclide symbol notation, but appears as a subscript to the right of the element symbol. Nuclides that have the same neutron number but a different proton number are called isotones. The various species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides. Each nuclide is denoted by chemical symbol of the element (this specifies Z) with tha atomic mass number as supescript. Therefore, we cannot determine the neutron number of uranium, for example. We can determine the neutron number of certain isotope. For example, the neutron number of uranium-238 is 238-92=146.
Neutron and Mass Numbers and Nuclear Properties
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with slow neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of ‘paired spin’. On the other hand, nuclei with an odd number of protons and neutrons are mostly unstable.
Neutron and Atomic Numbers and Nuclear Stability
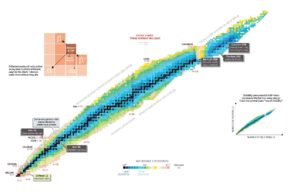
Nuclear stability is a concept that helps to identify the stability of an isotope. To identify the stability of an isotope it is needed to find the ratio of neutrons to protons. To determine the stability of an isotope you can use the ratio neutron/proton (N/Z). Also to help understand this concept there is a chart of the nuclides, known as a Segre chart. This chart shows a plot of the known nuclides as a function of their atomic and neutron numbers. It can be observed from the chart that there are more neutrons than protons in nuclides with Z greater than about 20 (Calcium). These extra neutrons are necessary for stability of the heavier nuclei. The excess neutrons act somewhat like nuclear glue. Only two stable nuclides have fewer neutrons than protons: hydrogen-1 and helium-3.
Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge. These two forces compete, leading to various stability of nuclei. There are only certain combinations of neutrons and protons, which forms stable nuclei.
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus. If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay. Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, gamma decay or electron capture. Many other rare types of decay, such as spontaneous fission or neutron emission are known.
Atomic Mass of Oxygen
Atomic mass of Oxygen is 15.9994 u.
The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance.
The size and mass of atoms are so small that the use of normal measuring units, while possible, is often inconvenient. Units of measure have been defined for mass and energy on the atomic scale to make measurements more convenient to express. The unit of measure for mass is the atomic mass unit (amu). One atomic mass unit is equal to 1.66 x 10-24 grams. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
For 12C the atomic mass is exactly 12u, since the atomic mass unit is defined from it. For other isotopes, the isotopic mass usually differs and is usually within 0.1 u of the mass number. For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63 and an isotopic mass in its nuclear ground state is 62.91367 u.
There are two reasons for the difference between mass number and isotopic mass, known as the mass defect:
- The neutron is slightly heavier than the proton. This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12C with equal numbers of protons and neutrons.
- The nuclear binding energy varies between nuclei. A nucleus with greater binding energy has a lower total energy, and therefore a lower mass according to Einstein’s mass-energy equivalence relation E = mc2. For 63Cu the atomic mass is less than 63 so this must be the dominant factor.
Note that, it was found the rest mass of an atomic nucleus is measurably smaller than the sum of the rest masses of its constituent protons, neutrons and electrons. Mass was no longer considered unchangeable in the closed system. The difference is a measure of the nuclear binding energy which holds the nucleus together. According to the Einstein relationship (E=mc2), this binding energy is proportional to this mass difference and it is known as the mass defect.
See also: Atomic Mass Number – Does it conserve in a nuclear reaction?
Hydrogen
Nonmetals
Helium
Noble gas
Lithium
Alkali metal
Beryllium
Alkaline earth metal
Boron
Metalloids
Carbon
Nonmetals
Nitrogen
Nonmetals
Oxygen
Nonmetals
Fluorine
Nonmetals
Neon
Noble gas
Sodium
Alkali metal
Magnesium
Alkaline earth metal
Aluminium
Post-transition metals
Silicon
Metalloids
Phosphorus
Nonmetal
Sulfur
Nonmetal
Chlorine
Nonmetal
Argon
Noble gas
Potassium
Alkali metal
Calcium
Alkaline earth metal
Scandium
Transition metals
Titanium
Transition metals
Vanadium
Transition metals
Chromium
Transition metals
Manganese
Transition metals
Iron
Transition metals
Cobalt
Transition metals
Nickel
Transition metals
Copper
Transition metals
Zinc
Transition metals
Gallium
Post-transition metals
Germanium
Metalloids
Arsenic
Metalloids
Selenium
Nonmetal
Bromine
Nonmetal
Krypton
Noble gas
Rubidium
Alkali metals
Strontium
Alkaline earth metals
Yttrium
Transition metals
Zirconium
Transition metals
Niobium
Transition metals
Molybdenum
Transition metals
Technetium
Transition metals
Ruthenium
Transition metals
Rhodium
Transition metals
Palladium
Transition metals
Silver
Transition metals
Cadmium
Transition metals
Indium
Post-transition metals
Tin
Post-transition metals
Antimony
Metalloids
Tellurium
Metalloids
Iodine
Nonmetal
Xenon
Noble gas
Caesium
Alkali metals
Lanthanoids
Hafnium
Transition metals
Tantalum
Transition metals
Tungsten
Transition metals
Rhenium
Transition metals
Osmium
Transition metals
Iridium
Transition metals
Platinum
Transition metals
Gold
Transition metals
Mercury
Transition metals
Thallium
Post-transition metals
Lead
Post-transition metals
Bismuth
Post-transition metals
Polonium
Post-transition metals
Astatine
Metalloids
Radon
Noble gas
Francium
Alkali metal
Radium
Alkaline earth metal
Actinoids
Rutherfordium
Transition metal
Dubnium
Transition metal
Seaborgium
Transition metal
Bohrium
Transition metal
Hassium
Transition metal
Meitnerium
Darmstadtium
Roentgenium
Copernicium
Nihonium
Flerovium
Moscovium
Livermorium
Tennessine
Oganesson
Lanthanum
Lanthanoids
Cerium
Lanthanoids
Praseodymium
Lanthanoids
Neodymium
Lanthanoids
Promethium
Lanthanoids
Samarium
Lanthanoids
Europium
Lanthanoids
Gadolinium
Lanthanoids
Terbium
Lanthanoids
Dysprosium
Lanthanoids
Holmium
Lanthanoids
Erbium
Lanthanoids
Thulium
Lanthanoids
Ytterbium
Lanthanoids
Lutetium
Lanthanoids
Actinium
Actinoids
Thorium
Actinoids
Protactinium
Actinoids
Uranium
Actinoids
Neptunium
Actinoids
Plutonium
Actinoids
Americium
Actinoids
Curium
Actinoids
Berkelium
Actinoids
Californium
Actinoids
Einsteinium
Actinoids
Fermium
Actinoids
Mendelevium
Actinoids
Nobelium
Actinoids
Lawrencium
Actinoids
–